Download All Country Data
Countries need strong laboratory systems to find, rapidly stop and prevent epidemics. But the COVID-19 pandemic forced countries to scramble to determine how to mitigate the impact of a new and contagious virus. One important facet of containing any infectious disease threat is strengthening testing capacity. Through testing, infected people can learn their status and isolate, and public health officals can trace their contacts to stop the spread. This requires strong coordination among subnational and national laboratory networks, a trained laboratory workforce and quality control of tests.
In Africa, public health officials realized there were gaps preventing countries from scaling up COVID-19 testing. Countries lacked lab coordination at the national level; lab workers lacked technical guidance, knowledge and supervision; tests lacked quality assurance; and many faced stockouts or shortages. When COVID-19 hit, only two countries—Senegal and South Africa—had testing capability. Officials sought to strengthen the capacity for quality COVID-19 testing across the continent.
Scaling Up Testing
In early March 2020, the Africa Centres for Disease Control and Prevention (ACDC) released its Joint Continental Strategy. Based on consultations with all African Union health ministers, the paper detailed ways to mount an effective continent-wide response to COVID-19. A central tenet was to “ensure quality-assured testing for COVID-19 diagnosis.” With this in mind, the African Society for Laboratory Medicine (ASLM), on behalf of ACDC, worked to strengthen 10 countries’ national capacities for surge testing. Resolve to Save Lives (RTSL), supported the initiative by funding technical expert positions at ACDC.
The ASLM project targeted 10 countries: Burkina Faso, Cameroon, Ethiopia, Gabon, Gambia, Ghana, Kenya, São Tomé and Príncipe, Uganda and Zimbabwe, from June 2020 to March 2021. To scale up testing capacity across these areas, the initiative provided funding and staff training that helped activate 251 new COVID-19 molecular testing labs for analyzing PCR test results. In Zimbabwe, the number of labs jumped from a baseline of six in June 2020 to 96 in March 2021 — a 1,500% increase.
The program also expanded COVID-19 testing by repurposing tools used for other infectious diseases, such as HIV and tuberculosis, and rolling out rapid diagnostic tests (RDTs) across six of the 10 countries. The use of RDTs was beneficial in countries that lacked PCR tests, which were initially harder to develop than RDTs. For example, in Cameroon, officials assessed the efficacy of four RDTs and found their diagnostic capacity strong enough to use widely in the absence of the more accurate PCR tests.
Between June 2020 and March 2021, the initiative facilitated about 1.9 million PCR tests across the 10 countries, comprising about 25% of the total number of COVID-19 tests conducted within this region during this period.

Researchers in one of COVID-19 molecular testing laboratories at Ethiopian Public Health Institute
Quality Assurance for COVID-19 Tests
Although the scale-up of testing was impressive, the project’s stakeholders knew it would only matter if the tests were of high quality. Thus, the initiative also worked to promote quality assurance in the COVID-19 tests it helped to administer. Using External Quality Assessment (EQA) schemes, the project promoted the comparison of a lab’s testing to a source outside the lab – national or international. With additional support from the CDC Foundation, this part of the initiative also targeted the Central African Republic, Chad and Burundi.
Phlebologists and lab technicians needed guidance to ensure that proper and standardized testing protocols were maintained. In response, ASLM officials conducted trainings with nearly 3,000 people on a range of testing-related topics, including proper sample collection and transport, laboratory quality management systems and national COVID-19 testing guidelines (see figure below). The project also developed 43 documents to support strategies and guide best practices for national lab systems.
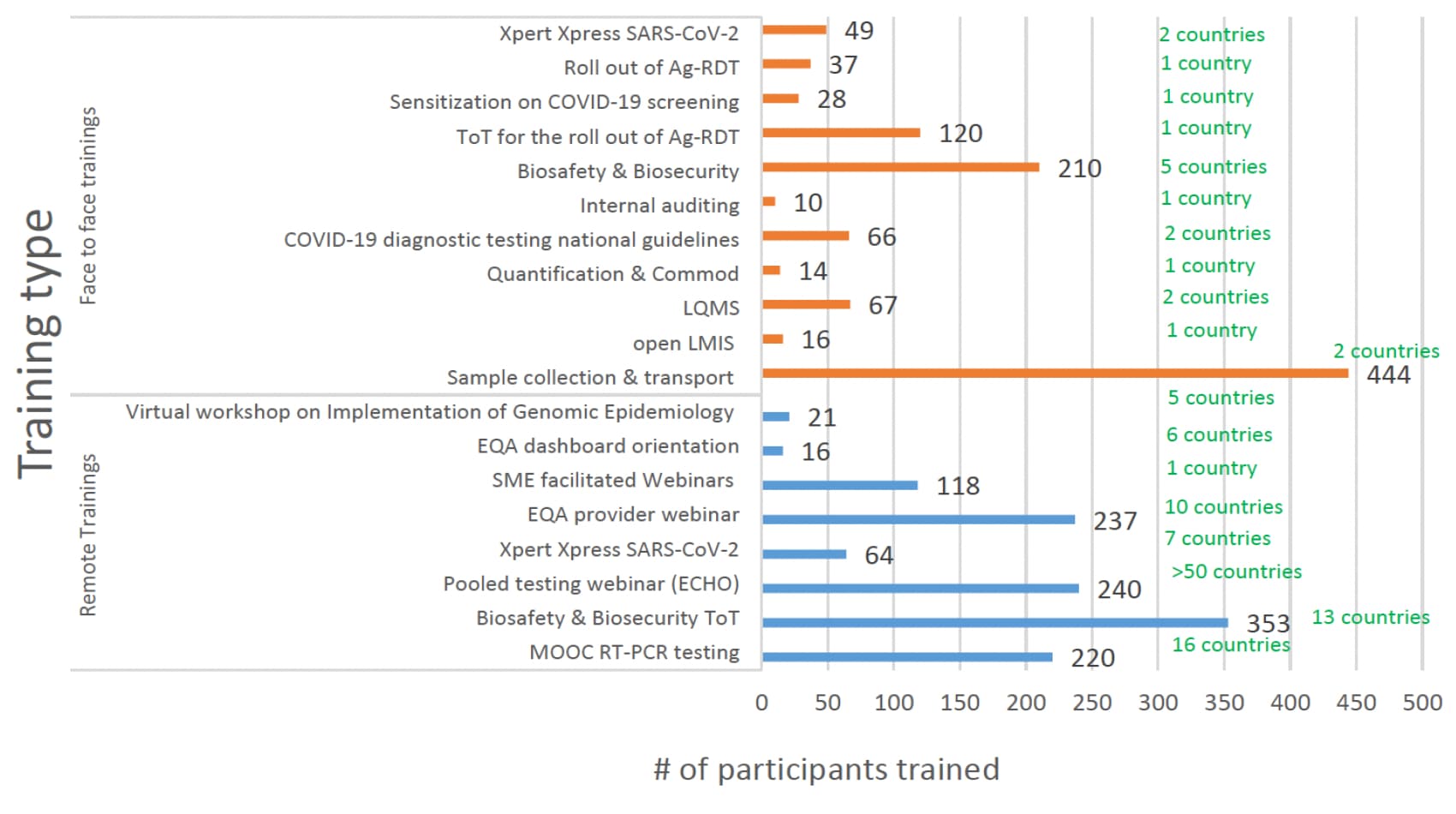
Dashboards were developed and implemented in six of the 10 countries to provide public, at-a-glance views of EQA participation and progress.
“To enroll our COVID-19 testing laboratories in External Quality Assurance programs such as Panels Testing and Blinded Retesting has helped us to ensure the quality of testing performance and identifying gaps for continuous quality assurance,” said Wondimeneh Liknaw, National EQA Assessment Coordinator at the Ethiopian Public Health Institute.
By the end of the initiative, the total number of testing sites had increased from 86 to 331. Of these sites, 245, or 74%, were enrolled in an international EQA scheme for COVID-19. Of the labs enrolled, at least 80% passed international standards for EQA. Notably, 100% of Uganda’s molecular testing labs enrolled in the national EQA scheme.
“In spite of a continuous internal quality control system in place, the concordant results obtained from this External Quality Assurance program, together with other laboratories, gives us assurance on the quality of results delivered to clients,” said Dr. Joseph Fokam, Chief of Service and Senior Scientist at Chantal Biya International Reference Centre (CIRCB) in Cameroon.
The initiative also encouraged cross-country peer-to-peer support. For example, trainees from Cameroon and Gabon collaborated to deliver a training on quality management systems. Representatives from Burkina Faso, Ethiopia, Uganda and Burundi held a virtual meeting to share challenges and successes from national EQA schemes. A WhatsApp group with 148 members from 11 countries provided further opportunities to share lessons learned.
“This initiative is an insightful model for sharing experience with peers and for training the next generation of African health scientists to respond against current and future outbreaks,” said Yenew Kebede Tebeja, the head of ACDC’s lab division. “Countries reached out to each other to share best practices and lessons learned to help make quality testing available across the African continent. Collaboration was the key to this program’s success.”






