Data insight:
Syndromic surveillance should be explored as an early signal for COVID-19
Syndromic surveillance is an innovative surveillance technique designed to detect illness clusters early, before diagnoses are confirmed and reported to public health agencies, and to mobilize a rapid response, thereby reducing morbidity and mortality. In the United States, syndromic surveillance systems were initially implemented in the early 21st century out of concern for intentional biologic and chemical events. Since then, they have evolved to be a critical component of overall public health surveillance, providing timely information on potential disease outbreaks and disease trends. The National Syndromic Surveillance Program allows for the timely exchange of syndromic data including clinical and contextual data. In New York City, the Syndromic Surveillance Unit collects data from all 53 emergency departments (ED) (100% of all ED visits), including chief complaint – that is, the reason for visit in the patient’s own words as recorded by triage staff, and automatically identifies whether a patient falls into one of 5 syndromes. Two of these syndromes could be used to detect an increase in complaints associated with COVID-19:
- Influenza-like illness (ILI) syndrome includes mention of fever, flu and cough or sore throat
- Respiratory includes mention of bronchitis, chest cold, chest congestion, chest pain, cough, difficulty breathing, pneumonia, shortness of breath, and upper respiratory
Data on the number and proportion of each syndrome is publicly available and updated daily. This type of information can be used as an early signal for COVID-19 if an increase in the number or proportion of visits due to ILI and Respiratory syndromes is driven by symptomatic COVID-19 patients.
Looking back at ILI data from NYC from March 2020, the proportion of ED visits due to both ILI and Respiratory syndromes started to rise in early March, which is atypical when compared to seasonal averages and occurred in the context of falling influenza rates. By the end of the first week of March there was a sustained increase in ILI indicators, when NYC reported 13 confirmed cases of COVID-19. By March 16 when officials closed New York schools, there were 4977 total confirmed cases of COVID-19. As jurisdictions consider when to tighten restrictions after periods of sustained COVID-19 suppression, syndromic surveillance data should be considered as an early indicator of the spread of infection. When the doubling time is as short as two days, every day of delay implementing physical distancing increases the number of cases, hospitalizations, and deaths by 50%. Systems for ILI and severe acute respiratory illness (SARI) around the world should be explored as tools for early detection of COVID-19.

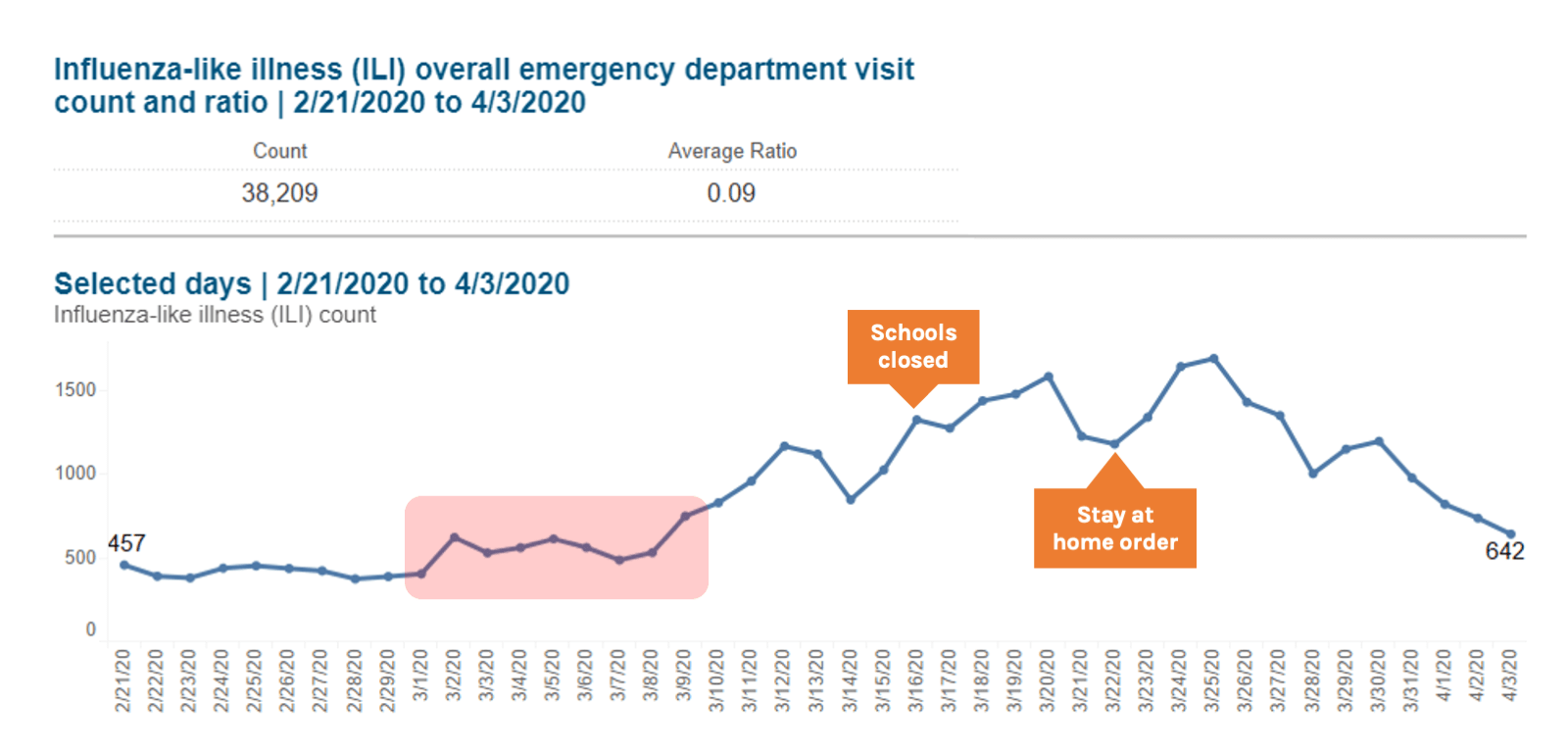
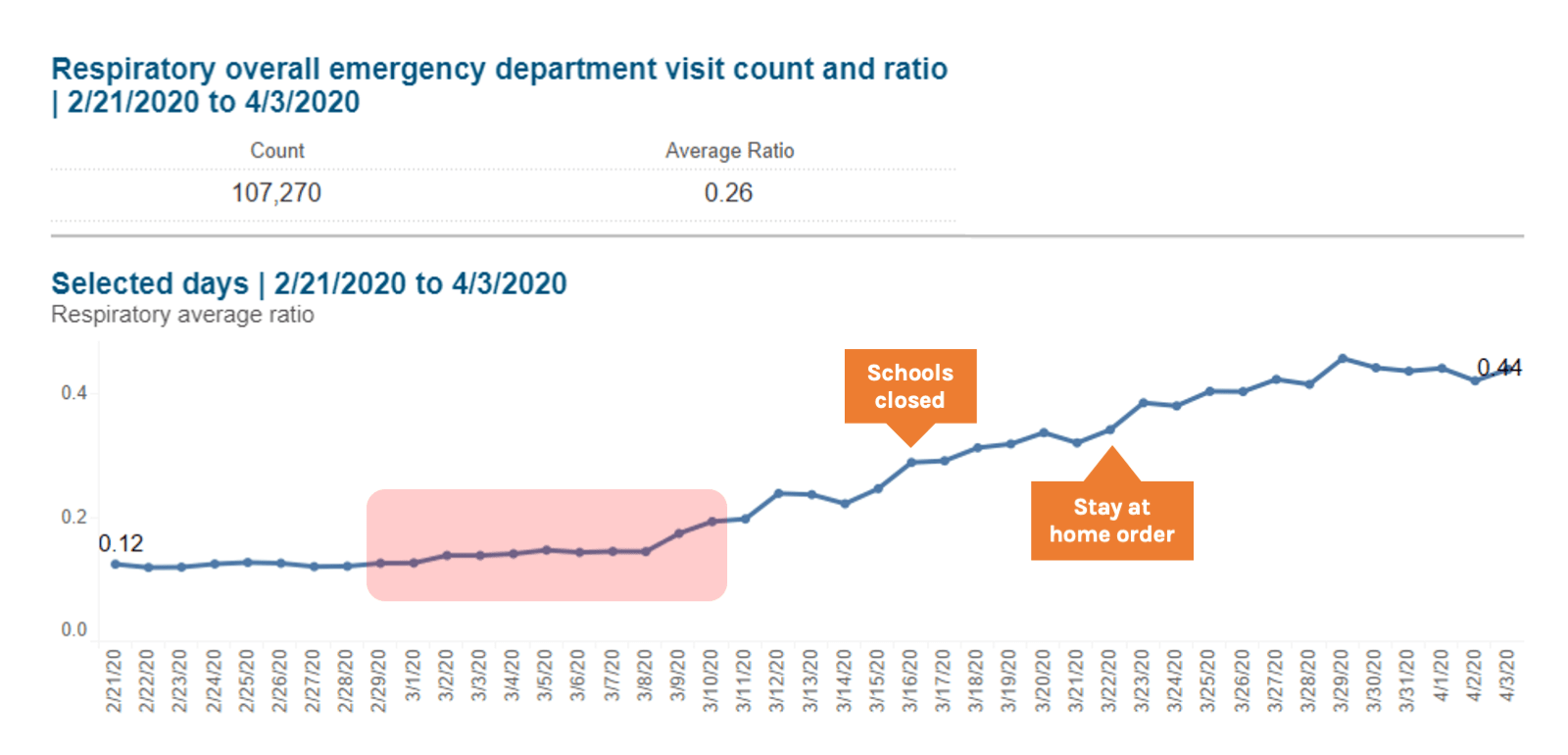
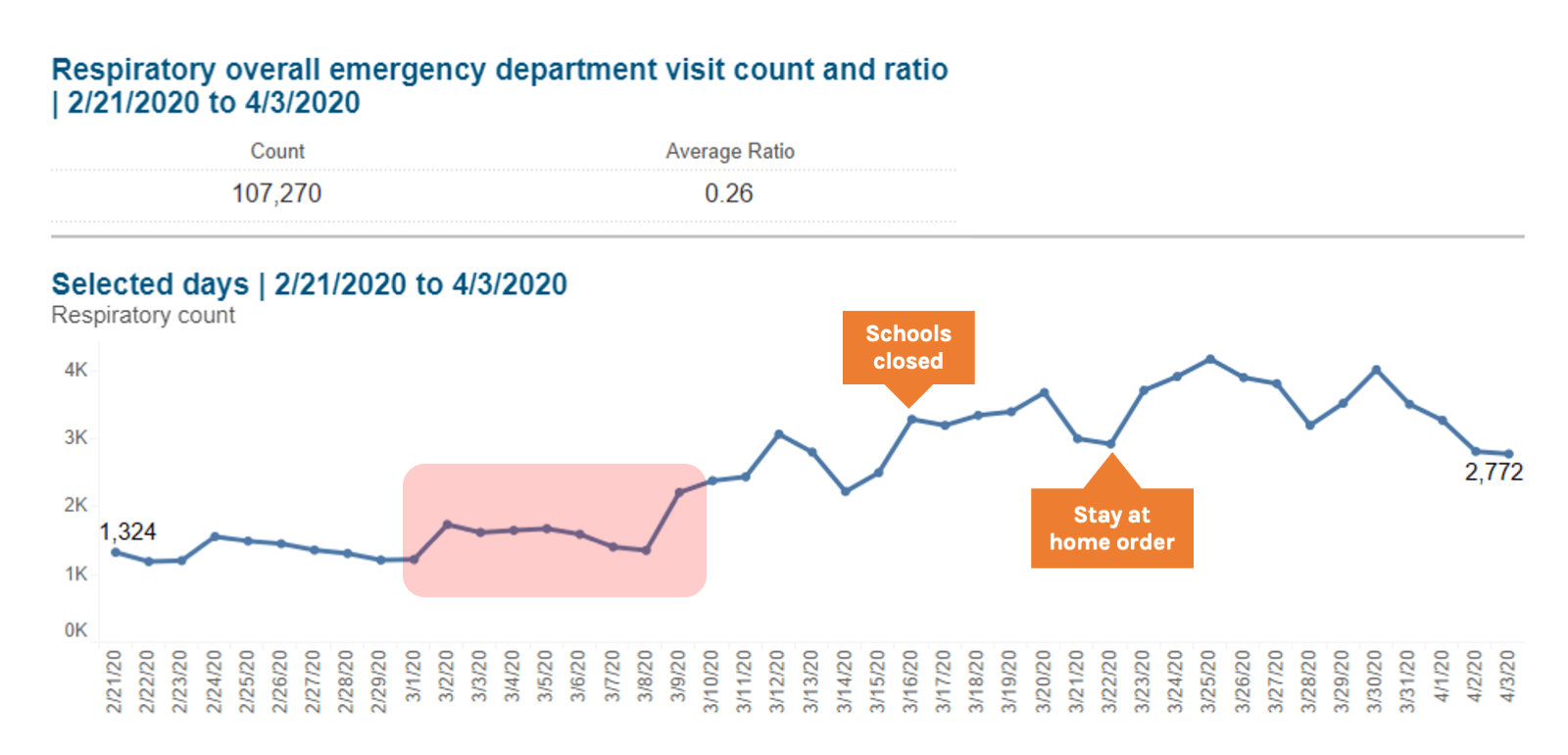
Source: New York City Syndromic Surveillance Data, accessed 3 April 2020
Face masks in the community
Several national and local governments with community transmission of COVID-19 have recommended the use of facemasks by everyone in the community regardless of whether they are sick or well. The use of facemasks for symptomatic individuals is recommended by WHO for all influenza pandemics regardless of severity. A recent study also supports the use of surgical face masks to prevent coronavirus transmission from symptomatic individuals. Overall, there is consensus that those who work in healthcare and those who are sick should wear facemasks as they can prevent transmission of COVID-19. An increasing number of health facilities, including nursing homes, are adopting the practice of all staff and patients wearing a face mask at all times, in an effort to protect health workers and patients.
Evidence to support the use of face masks for those who are not symptomatic is limited. The use of face masks by the general public was only conditionally recommended by WHO only in severe influenza pandemics, as there was no evidence that they were effective in reducing transmission but there was mechanistic plausibility for potential effectiveness. In the current context of a global shortage of both respirators (e.g. N95) and surgical masks which are needed for healthcare workers and the sick, the only reasonable recommendation that can be made is for those without symptoms to wear cloth masks, scarves or homemade masks. There is variation in the community in the quality of these facial coverings, material used, fit, and how and how often they are cleaned. There is very limited data on the efficacy of cloth masks. There are some small studies (1,2,3) showing that cloth barriers provide some level of marginal protection against particles which can contain viruses. If they are worn regularly, they must also be changed and washed often. And if a covering gets wet, even from the moisture emitted when a person exhales, the fabric could be more likely to transmit the virus. One randomized trial compared medical masks, cloth masks and usual practice in 1607 hospital health care workers over a 4-week period. Cloth masks were 2 layer cotton masks which participants were asked to wash daily with soap and water. They found that the highest rates of influenza-like illness were in the cloth mask group (Relative Risk 13.0 (95% CI 1.7-100.1) compared to the medical mask arm. They were also higher in the cloth mask group compared to the usual practice group. Cloth masks also had higher rates of laboratory confirmed virus in participants (RR 1.72 95% CI (1.01-2.94) compared with the medical mask group. Lastly, penetration of cloth masks by particles was almost 97% compared to 44% in medical masks. The authors cited moisture retention, reuse of cloth masks and poor filtration as potential reasons for this observed increased risk of infection.
There is no new strong scientific evidence that they are useful. As we learn more about the role of asymptomatic infection and transmission of COVID-19, our understanding of the benefit of basic physical barriers such as cloth masks may change. Currently, expert groups are looking at the risks and benefits of facial coverings in the community and will make recommendations which should include specific guidance on how to maximize their effectiveness. Regardless, for the general public, the public health benefit of wearing a mask is still not clear; even if someone has a mask, if they don’t use it properly, it won’t be effective, and masks should not replace hygiene practices and physical distancing. As WHO Health Emergencies Executive Director Mike Ryan said on 3 April 2020 “It is not the ideal solution, but it should be considered in the context of a comprehensive strategy to control the disease, it should be considered in terms of the type of transmission that is happening, how intense transmission is at community level, the circumstances of transmission, resources available, and we must preserve medical, surgical, and respirator masks for front line workers….That doesn’t negate the need for hand washing, physical distancing, for people to stay at home if there is an order, for everyone to protect themselves and to protect others.”
Pets and COVID-19
Our understanding of pets and COVID-19 is based on media reports, one preprint serologic study of cats and official statements from Hong Kong’s Agricultural, Fisheries and Conservation Dept. One article examined the three reports of pets in homes of COVID-19 patients in which these pets tested positive by RT-PCR. This included two dogs in Hong Kong (one died shortly after release from quarantine) from different households, both asymptomatic and one cat in Belgium (positive samples collected by ill owner in self-quarantine). The dog who died was a 17-year-old Pomeranian (in calendar years, not dog years) who developed an antibody response, but was in poor health to begin with and the death was not considered related to SARS-CoV2. The other dog survived and was 2-year-old German Shepherd. The cat reportedly had a prior history of GI and respiratory illnesses and developed vomiting, diarrhea and difficulty breathing 7 days after owner returned from Italy. Those symptoms resolved within 9 days. In a study of sera from 102 cats in Wuhan (a mix of pets, hospital cats and stray cats) after the COVID-19 outbreak, they found that 145 (14.2%) samples were positive for SARS-CoV-2 by ELISA and 11 of those had SARS-CoV-2 antibodies. Three cats which were pets of humans infected with COVID-19 had the highest titers. Overall, they found evidence that the cat population in Wuhan was infected with COVID-19, but no known evidence of illness or transmission from pets to humans. They recommended that humans and companion animals keep a suitable distance and further studies to evaluate COVID-19 and domestic animals. Current CDC guidance states there is no evidence that companion animals, including pets, can spread COVID-19. The guidance also notes that there is no evidence to suggest that imported animals or animal products pose a risk of spreading the 2019 novel coronavirus in the United States. They do note that if you are sick with suspected or confirmed COVID-19, you should avoid contact with your pet including, petting, snuggling, being kissed or licked, and sharing food. If you must care for your pet or be around animals while you are sick, wash your hands before and after you interact.
How common is asymptomatic infection?
Asymptomatic infection is general term that at a given point in time can include those who will later go in to develop symptoms (pre-symptomatic) and those who will never have noticeable symptoms (asymptomatic). Several recent studies examining the Diamond Princess cruise ship, long-term care facility in Seattle, WA, USA, Japanese nationals evacuated from Wuhan, and areas with robust testing (Iceland and Vo, Italy) indicate that the actual and estimated asymptomatic ratios range from 18%-50% (proportion of all cases that are asymptomatic). Furthermore, asymptomatic infection is likely to be age-related: a large proportion of children with infection appear to have few or no symptoms. In one study of 36 children with COVID-19, 10 (28%) were asymptomatic and all of the remaining 26 children had mild or moderate symptoms.
For more information, see our asymptomatic infection supplement
Can someone get re-infected?
Several media reports (1, 2) came out about possible cases of SARS-CoV-2 reinfection. More specifically, people with confirmed infection in China who had recovered and tested negative subsequently tested positive. Details on these cases are sparse and may well be related to issues with the process of taking the sample (which can vary) and the performance of diagnostic tests, some of which have a high false negative rate. This means that the negative test might not have truly been negative, but more a result of an inaccurate test or inadequate specimen. Another possibility is that the subsequent test was a false positive and picked up remnants of residual virus. Other coronaviruses have been shown to generate a sustained antibody response to natural infection. In both SARS-CoV-1 and MERS this antibody response lasted beyond a year. Per Denis Nash, epidemiological studies are needed in outbreak settings that examine the short- and longer-term risk of reinfection and development of symptoms among those with serologic evidence of SARS/COV2 infection. It is also possible that some people continue to harbor the virus in their upper respiratory tract for some weeks after illness, as occurs with the common cold coronaviruses, for which there are short-term carrier states which may be important in facilitating the continuous spread of these viruses. In one 2006 study of 217 well-protected health care workers in Taiwan, 25 (11.5%) were found to be colonized with SARS-CoV-1 without seroconversion and may have been capable of transmitting the virus to susceptible individuals.
Articles
Epidemiology
Detection of SARS-CoV-2 Among Residents and Staff Members of an Independent and Assisted Living Community for Older Adults — Seattle, Washington, 2020
(MMWR 3 April 2020)
- This article examined all 80 residents and 62 staff members of a Seattle independent and assisted living facility where stringent preventive measures were implemented after two residents were hospitalized with COVID-19 infection earlier in the month.
- Testing of all residents and staff members only found few cases of COVID-19 (probably due to the strict measures in place). Three of four residents who had positive test results were asymptomatic.
- Symptom-based screening might not identify SARS-CoV-2 infections in independent and assisted living facility residents, underscoring the importance of adhering to CDC guidance to prevent COVID-19 transmission in all senior living communities
Rapid Sentinel Surveillance for COVID-19 — Santa Clara County, California, March 2020
(MMWR 3 April 2020)
- To rapidly understand the extent of COVID-19 in the community, four urgent care centers served as sentinel surveillance sites
- During March 5–14, among patients with respiratory symptoms, 23% had positive test results for influenza. Among a subset of patients with negative test results for influenza, 11% had positive test results for COVID-19.
- As a result of these data and an increasing number of cases with no known source of transmission in Santa Clara County, the county initiated a series of community mitigation strategies to slow the spread of SARS-CoV-2 as early as March 9 (cancellation of mass gatherings >1,000 people)
Presymptomatic Transmission of SARS-CoV-2 — Singapore, January 23–March 16, 2020
(MMWR 1 April 2020)
- The authors reviewed all COVID-19 cases in Singapore through March 16 to determine whether presymptomatic transmission might have occurred.
- Seven COVID-19 epidemiologic clusters in which presymptomatic transmission likely occurred were identified, and 10 such cases within these clusters accounted for 6.4% of the 157 locally acquired cases.
- Notably, one cluster involved likely contact transmission on a church seat (captured by close-circuit camera), and another cluster occurred in a singing class.
- The authors suggest that containment measures should account for the possibility of presymptomatic transmission by including the 72 hours before symptom onset when conducting contact tracing.
Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility — King County, Washington, March 2020
(MMWR 3 April 2020)
- A COVID-19 outbreak spread rapidly in a skilled nursing facility, and 16 days after initial introduction of SARS-CoV-2 into the facility, 30% of residents were found to be infected (despite early adoption of infection prevention and control measures).
- Of the 23 residents who tested positive, 10 were symptomatic and 13 asymptomatic at the time of testing. Of the 13 asymptomatic, 10 went on to develop symptoms (and were reclassified as presymptomatic) but 3 (3/23 = 13%) remained asymptomatic.
- Symptom screening could initially fail to identify approximately one half of SNF residents with SARS-CoV-2 infection. There was no statistically significant difference in distribution of CT values (indicator of viral RNA present by RT-PCR) among the symptom status groups.
Response
Impact of school closures for COVID-19 on the US health-care workforce and net mortality: a modelling study
(Lancet Public Health, 3 April 2020)
- The authors aimed to measure child-care obligations for US health-care workers arising from school closures and assess how important the contribution of health-care workers would have to be in reducing mortality for their absenteeism due to child-care obligations, to undo the benefits of school closures in reducing the number of cases.
- They estimate that 1 in 7 frontline medical workers may need to miss work to care for their children if US schools were to close to reduce the spread of COVID-19.
- In their models, they found that school closures, in the absence of other child-care options, could either increase COVID-19 mortality through a health-care labor force reduction pathway or decrease COVID-19 mortality through a case reduction pathway.
- Based on the data they have, they could not provide a clear indication of which pathway will be dominant.
Estimating the number of infections and the impact of non-pharmaceutical interventions on COVID-19 in 11 European countries
(Imperial College MRC, 30 March 2020)
- The authors looked at 11 European countries which have implemented unprecedented non-pharmaceutical interventions including case isolation, the closure of schools and universities, banning of mass gatherings and/or public events, and most recently, widescale physical distancing including local and national lockdowns.
- Int their models, they assume that each intervention has the same effect on the reproduction number across countries and over time.
- With current interventions remaining in place to at least the end of March, they estimate that interventions across all 11 countries will have averted 59,000 deaths up to 31 March [95% credible interval 21,000-120,000].
- They estimate that across the 11 European countries, the average Ro has declined 63% from 3.87 to 1.43.
FAQs
How does COVID-19 relate to blood type?
It has been reported that certain blood types may be associated with an increased risk of COVID-19. This is based on a non-peer reviewed study of 2,173 patients in Wuhan, China which showed that blood group A was associated with a higher risk for acquiring COVID-19 compared with non-A blood groups, whereas blood group O was associated with a lower risk for the infection compared with non-O blood groups. More specifically, the proportion of blood group A in patients with COVID-19 was significantly higher than that in a group of 3,694 controls from a recent survey in Wuhan. the general population, being 38% in the former vs 32% in the later (P < 0.001) The observed higher risk for blood group A was not replicated in a similar comparison of 285 COVID-19 patients and 23,368 controls in Shenzen, China. The authors of this study acknowledge that this is an early study with limitations and should not guide clinical practice. Others note that the authors did not provide an explanation for this observation and the findings should not change the behavior of people with certain blood types. Those with Type A or Type O blood should continue to follow guidance to prevent infection (as should those of any blood type).
Suggested citation: Cash-Goldwasser S, Kardooni S, Cobb L, Bochner A, Bradford E and Shahpar C. In-Depth COVID-19 Science Review March 28 – April 3, 2020. Resolve to Save Lives. 2020 April 6. Available from https://preventepidemics.org/covid19/science/review/







