Immunity Passports
Our May 10-17 Weekly Science Review examined whether immunity passports could be issued to people with antibodies to SARS-CoV-2, the virus that causes COVID-19. At the time, expert guidance recommended against such immunity passports because of fundamental gaps in our understanding of the immunological response to the virus, along with serious legal and ethical issues. More than six months later, national regulatory bodies have already authorized several vaccines and other candidate vaccines are close behind in clinical trials. As waves of people are vaccinated, policymakers will need to provide the public with clear guidance about activities that are safe for different people. Immunity passports are a potential tool to enable us to shift from measures based on population-level risks to those more fine-tuned to individual-level risks.
Immunity passports could potentially touch every aspect of modern life that has been affected by COVID-19. They might be required to work in certain professions or facilities (e.g., nursing homes, hospitals, commercial airplanes, meatpacking plants, schools, and essential businesses). Immunity passports might be required to live in certain facilities (e.g., long-term care, government-subsidized, university housing, certain apartments, etc.) or, eventually, for in-person attendance at public and private schools. Travel, whether domestic, interstate or international, might be conditional on immunity (e.g., public transportation passes, cruise or airline tickets, membership to ride-sharing apps). Documented immunity might be required to participate in leisure activities such as restaurant dining, shopping or working out, or to attend mass gatherings such as concerts, sporting events and weddings. In addition to granting permission to work or attend an event, an immunity passport could potentially exempt the holder from certain public health and social measures, such as mask mandates, physical distancing requirements, quarantine or stay-at-home orders.
The more society opens up to a person with immunity passports, the more valuable these passports would become. If implemented effectively, passports could serve as a powerful incentive for vaccine uptake. Consequently, the perceived value also increases the risk that the passports could become vehicles for inequality and discrimination as long as disparate access remains to vaccines and testing. Privacy and data security lapses could also lead to devastating health and social consequences, as could the circulation of counterfeit passports. The scientific, legal, administrative and ethical issues should be analyzed and openly discussed now so that any immunity passport program keeps pace with vaccine administration efforts and our understanding of immunity to COVID-19.
Immunity Passport = Certificate + License
From a legal standpoint, terminology is crucial. The term “immunity passport” conflates licenses and certificates, which are related but distinct legal tools. A license is a permit, or permission, from an authority to engage in a particular activity. A certificate, by contrast, is a document attesting to a set of facts. An effective immunity passport will include both elements: (1) permission to engage in an otherwise restricted activity, based on (2) a trustworthy certification that the person has the expected level of protection.
Travel documents serve as a helpful illustration. A country might grant a foreigner permission to enter the country by issuing them a visa, a type of license, if they fulfill certain requirements. This visa is associated with the foreigner’s passport, which is essentially a certificate issued by their home country attesting they are a national of that country and that they are who they claim to be. The combination of the validation from the home country and permission from the host country allow the person to travel. Although the passport is designed as a travel document, passports are also used by private institutions such as banks to confirm the identity of customers or employees. The International Civil Aviation Organization produces standards on the design, biometrics and procedures for the issuance of passports and other elements of passports. A web of national laws and international agreements then regulate global entry and exit rules.
When determining whether to pursue an immunity passport program, policymakers at all levels should consider the implications of various combinations of licenses and certificates. Access to a high-risk setting may require a higher degree of certainty, for example requiring nursing home visitors to maintain a valid certificate of vaccination issued by an approved clinic. In a lower-risk setting, lower certainty may be acceptable, such as a private elementary school allowing entry to campus with a doctor’s note asserting full recovery from COVID-19. Legal and other issues are more likely to arise when there is discord between the two, such as high-risk settings that allow nearly everyone access or a low-risk setting that has unnecessarily burdensome restrictions.
Licenses
As noted above, a license is a permit, or permission, from an authority to engage in a particular activity. Immunity-based licenses present an enticing method to ratchet down blanket protective measures without increasing health risks. Governments, employers, or other private entities could issue immunity-based licenses within their realm of control.
Government-issued licenses: Local and national governments worldwide have imposed public health and social measures to reduce community spread of COVID-19, including mask mandates, distancing requirements, mass gathering limitations, quarantine, curfews and stay-at-home orders. Under national and international law, when these restrictive measures cause incidental infringement on rights, such as freedom of movement or religion, the measures cannot be more invasive or intrusive than reasonably available alternatives that would achieve the appropriate level of health protection (Article 43 of the IHR). The Supreme Court of the United States recently held that restrictions that infringe on free exercise of religion would be held to strict scrutiny, meaning that restrictions must be narrowly tailored to achieve a compelling state interest. Arguably the compelling state interest for enforcing these public health measures erodes if there is no longer a legitimate risk to the individuals or their community. It is unethical, and potentially illegal in some countries, for governments to maintain these overly expansive restrictions if less burdensome alternatives—such as a requirement to prove immunity to COVID-19—could achieve the same health protection.
Governments could also create an environment that is more conducive to immunization uptake by mandating immunization as a condition for engaging in certain activities, such as employment, education, traveling or enrolling in child care. Systematic reviews have shown that imposing school vaccination mandates is associated with increased vaccine coverage among children. Certain vaccinations are already required for employment or education in many settings. Beginning in 2016, Australians were required to show proof of their children’s adherence to immunization schedules before gaining access to preschool admission (“No jab, no play”) and family financial assistance payments (“No jab, no pay”). The program is credited with increasing vaccine uptake to record highs. Medical exemptions are permitted, but exemptions for religious or personal beliefs are not.
Employee licenses: Many employers have been exercising a duty of care to their staff and customers by imposing COVID-19 restrictions that are stricter than local law requires. There are major advantages if employers can relax these restrictions for immune employees without increasing risk to others. Employers can legally impose generally applicable health and safety standards, such as distancing requirements, personal hygiene, or mask and other personal protective equipment mandates; however, other laws might hinder an employer’s attempts to distinguish employees with evidence of immunity from those without.
The Americans with Disabilities Act (ADA), for example, prohibits disability-related inquiries or medical examinations unless the tests are “job related and consistent with business necessity” and the employer has a reasonable belief, based on objective evidence, that an employee will pose a direct threat due to a medical condition. While the U.S. Equal Employment Opportunity Commission (EEOC) has issued guidance that someone with COVID-19 or symptoms of COVID-19 would present a direct threat, they have not gone so far as to conclude that susceptibility to COVID-19 is a direct threat. Under this guidance, job-related COVID-19 testing for active COVID-19 infection would be allowed, but antibody testing would not be allowed per CDC interim guidance, because antibody test results cannot be used to determine current infection status. Even when medical examination or inquiries are allowed under the ADA, they must be kept confidential.
Similarly, employers can require employees to get vaccinations, but an employee can avoid this requirement if a disability under the ADA or a “sincerely held” religious, ethical or moral belief under Title VII of the Civil Rights Act of 1964 prevents the employee from taking the vaccine. Employers must provide these exempt employees reasonable accommodation unless doing so would cause undue hardship to the business. Each situation is fact-specific and depends on the employee and the position, so there is considerable uncertainty about what accommodations are “reasonable.” In some situations, unvaccinated employees can fully and safely perform their job while, for example, wearing a mask or working from home. In other situations, these arrangements may not be practical. For these reasons, the EEOC suggests that employers encourage, but do not require, their employees to get vaccinated.
Other licenses: Other private entities might permit access to their services only to people who can prove immunity. Examples include mandating a health certificate before boarding an airplane or attending a concert or sports event. As with employers, businesses that are open to the public must provide reasonable accommodations to ensure equal access for individuals with disabilities. Denial of entry may not be allowed based on a single factor if other alternatives provide the same level of certainty. For example, a company might not be able to deny entry to a person who cannot be vaccinated (for medical, religious or supply reasons) if that person could show they have another form of immunity, recently tested negative, or could protect themselves or others by wearing personal protective equipment and distancing themselves from others.
Licenses could facilitate reopening aspects of public life while keeping risk minimal. However, success depends on trustworthy proof of immunity.
Certificates
A certificate is a document attesting to a set of facts—for example, that a person was vaccinated and where and when the vaccination occurred. An immunity certificate should not specify the level of protection that may be conferred by vaccination or previous infection. This is the case for certificates of vaccination against other diseases, which state that vaccines were given but not the extent to which the vaccine recipient is protected. As for other diseases, our understanding of the protection against COVID-19 that the bearer of a certificate of COVID-19 vaccination or previous infection may have should be informed by available scientific evidence.
As we have previously written, the immune response to a pathogen such as SARS-CoV-2 is complex, involving an array of organs, cells, proteins and molecules that signal among one another and attack invading pathogens in various ways. The adaptive immune response, which is trained to recognize pathogens and has “memory” of previous exposures, comprises B cells, which produce antibodies that impede the ability of viruses to infect new cells, and T cells, which directly kill infected human cells and also help direct other parts of the immune system.
It is likely that the vast majority of people have some level of immunity if they (a) have been vaccinated, (b) have recovered from a confirmed natural infection or (c) have had a positive antibody test. Although any of these three situations might indicate some level of immunity, many questions remain about the degree and longevity of immunity and level of protection. At least until our scientific understanding of immunity to and protection from COVID-19 matures, certificates should avoid anything that suggests that any of the above situations is a proxy for protective immunity, that immunity is long-lasting, or that any of the above situations guarantees that disease spread cannot occur. These inferences should be avoided because of scientific unknowns that fall into four general categories: 1) vaccination as a proxy for protection, 2) natural infection as a proxy for immunity, 3) antibodies as a proxy for protection, and 4) potential to transmit infection despite vaccination or natural infection.
1) Vaccination as a proxy for protection: Certification of vaccination is one way to indicate likely protection against COVID-19. With the development and delivery of vaccines for COVID-19 now at the forefront of the response, much remains to be seen about how well and for how long different vaccines will protect individuals and communities. No “real-world” effectiveness data are available yet for any of the vaccines already in use or for the dozens of vaccine candidates that are being studied. What is available is vaccine efficacy information, or information on how well a vaccine works under research conditions, from the vaccines that are in later study stages or have been approved or authorized for use. Currently, three vaccine manufacturers (Pfizer/BioNTech, Moderna, AstraZeneca) have released interim phase 3 efficacy data, with expected overall efficacy ranging from 70% to 95%, translating to a 70% to 95% reduction in the chance that a vaccinated person would get COVID-19 compared to an unvaccinated person. This means that even people who receive a highly effective vaccine may still get COVID-19, although at significantly lower rates than those who are unvaccinated. Complete data on the efficacy in different age groups, genders, races and ethnicities, pregnant women and women of reproductive age, or in those with certain medical conditions, are not yet available, nor are data on the duration of protection.
Available vaccine efficacy data from Pfizer/BioNTech does not offer information on the chance of asymptomatic infection with SARS-CoV-2 after vaccination. This is because trial participants were tested for COVID-19 only if they developed symptoms. Related to this, the extent to which vaccines reduce the chance of transmitting the infection to others, despite protection from symptomatic disease, is not yet clear. It is possible that some clarity may come from controversial “challenge studies,” in which people who have been vaccinated are intentionally exposed to the virus so researchers can study how the virus affects participants, monitor the level of virus in participants’ bodies, and learn more about the immune response to exposure after vaccination.
As it has only been months since late-phase vaccination trials began, there has not been enough time to evaluate with certainty the durability of protection offered by these vaccines in humans. These data will be forthcoming over the course of months and years. At present, inference about the durability of protection is based on what we know about immunity to other infections and from studies on immunity after natural infection with SARS-CoV-2. There will need to be ongoing observational studies after vaccination is rolled out in the community to determine how long protection lasts and if and when booster doses are needed.
Pfizer/BioNTech has released data on vaccine efficacy after just the first of two doses is given. After one dose, vaccine efficacy was by one estimate approximately half of the efficacy achieved after the second dose. In the U.S., a number of the candidate vaccines supported by Operation Warp Speed, the U.S. government’s program to rapidly produce and deliver millions of doses of safe and effective COVID-19 vaccine, require two doses to complete the vaccine series (one candidate vaccine may require a single dose). It is likely, as with the Pfizer/BioNTech vaccine, that other vaccines with two-dose regimens will offer some protection after the first dose. Decisions will need to be made, based on efficacy, about what is adequate for certification of vaccination and how this information is presented on any certificate. These questions are discussed in more detail below.
2) Natural infection as a proxy for protection: Another potential mechanism for documenting protection against COVID-19 is proof of recovery from a prior infection, such as a positive PCR test in the past. There are several examples of diseases for which a single natural infection may—more effectively than vaccination—lead to highly protective, long-lasting immunity. Examples include chickenpox and measles, the vaccines for which are given in two-dose schedules. People who have previously had chickenpox do not need to be vaccinated against chickenpox. People born before 1957, who lived through measles epidemics before the first measles vaccine was licensed in 1963, are presumed to be protected against measles. In another example, children’s immune responses generated by natural influenza infection during the 2009 influenza A/H1N1 pandemic were more robust than immune responses generated by vaccination.
However, lingering questions about immunity after natural infection with SARS-CoV-2 are important reasons why certification of vaccination may have different implications than certification of natural infection. First, many studies comparing the immune response generated by natural infection have been conducted in those with severe, or at least symptomatic, disease. This makes it difficult to draw conclusions about immunity among people with mild or asymptomatic infections. There is evidence that the immune response to mild infection may be less robust. Second, outside of a challenge trial, it is impossible to know the inoculum, or exposure dose, that has caused an infection. In contrast, the amount of immunologic stimulation given via vaccination is a well-controlled, studied amount. Different inoculums (or exposure to different clades, or strains, of the virus) may lead to different levels of immunity. Third, it is difficult to study the frequency of reinfection with SARS-CoV-2. There have been just a few documented cases ofreinfection with SARS-CoV-2 after an initial case of COVID-19 out of millions of COVID-19 cases. However cases of reinfection may be missed because asymptomatic reinfections may go undetected and because sophisticated diagnostic techniques are required to determine that two distinct infections have occurred. Fourth, the longevity of protection after natural infection is not known. Some studies have shown that antibodies against SARS-CoV-2 may quickly decline after infection, although other studies have shown that antibodies may persist for months. There is laboratory evidence of immunity lasting for years among some survivors of Middle Eastern Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS), which are caused by viruses related to SARS-CoV-2. However, the protection conferred by those immune responses is not clear as there are no documented cases of reinfection among SARS or MERS survivors. Further study on the correlation between natural infection with SARS-CoV-2 and protection against COVID-19 is needed.
Beyond this scientific uncertainty, another difficulty with certifying natural infection is that incentivizing infection is unethical. There are tremendous costs associated with natural infection, as some people who get COVID-19 develop serious complications and some die. Worldwide, 1.6 million people have died from COVID-19 to date. Decision makers should never establish policies that encourage healthy people to risk infection. At the same time, the 70 million people who have been diagnosed as confirmed cases should not be blocked from public life, especially if it is determined in the future that they have comparable immunity to people with licenses based on vaccination. The law, some argue, should treat immune people equally, regardless of how they acquired that immunity. Policymakers will need to weigh these competing arguments and determine what is appropriate for their communities.
3) Antibodies as a proxy for protection: A certificate might include the results of an antibody test—the most simple, affordable and accessible type of test for an immune response against a pathogen. Many tests for antibodies to SARS-CoV-2 have now been approved by the U.S. Food and Drug Administration. Antibody tests can help identify people who may have been infected with SARS-CoV-2 in the past. However, antibody test results may not correlate with protection against COVID-19. The presence or absence of antibodies may not always correctly represent infection status. If the test is conducted too early, it may miss antibodies that develop in the future. If conducted a long time after infection has occurred, antibodies may have already waned to undetectable levels. Antibody tests can give inaccurate results, with positive results when antibodies are not actually present (false positive) or negative results when antibodies actually are present (false negative). If antibodies are present, they likely confer some degree of protection. In one pre-print study in which thousands of health care workers were tested for SARS-CoV-2 over a six-month period, none of those with antibodies to SARS-CoV-2 developed symptomatic infections. However, the presence of antibodies does not guarantee protection. In the third phase of the Pfizer vaccine trial, at least one participant who tested positive for SARS-CoV-2 antibodies developed COVID-19. In the aforementioned study of health care workers, several of those with antibodies developed asymptomatic infections but at lower rates than health care workers without antibodies. Finally, as mentioned above, there are many parts of the immune response besides antibodies. Some people do not develop detectable antibodies after exposure to SARS-CoV-2 but develop T cell responses. Some COVID-19 survivors have robust T cell responses to SARS-CoV-2 months after infection despite waning antibody levels. The degree and longevity of protection associated with the presence of antibodies is not known.
Some countries presume immunity and/or protection based on a combination of test results. For example, Icelandcurrently exempts travelers from mandatory quarantine and testing requirements if the traveler presents documented evidence of a positive COVID-19 PCR test that’s at least 14 days old or results of an antibody test issued by an approved European lab. Hungary’s closed borders are open to people who can provide evidence they have recovered from COVID-19 by presenting evidence of a positive PCR test and then a negative PCR test conducted within the past six months.
4) Potential to transmit infection despite vaccination or natural infection: Another question surrounding immunity certificates is whether immunity, however it is acquired, prevents the spread of infection to others. Considerations are slightly different after vaccination and natural infection. Similar overarching principles are that a significant proportion of infections may be asymptomatic, and although those who never develop symptoms may transmit infection less frequently than those who become symptomatic, transmission from asymptomatic people does occur.
For those who have been vaccinated, the possibility of transmitting infection depends on whether people can still become infected after vaccination. Existing COVID-19 vaccines do not contain live virus, meaning that vaccination itself cannot cause COVID-19. For those who have been infected naturally, the possibility of transmitting infection depends on whether the primary infection is still transmissible and whether reinfection may occur. For the first question, studies have shown that among those that develop symptoms, peak viral shedding and peak infectiousness occurs from a couple of days before to a few days after symptom onset. Although prolonged viral shedding can occur(so that virus may be detectable by PCR), prolonged shedding of infectious virus (virus that is capable of infecting another person) for longer than 10 days after mild or moderate symptom onset has not been documented. This is the scientific basis for recommendations on the duration of quarantine and isolation. As above, reinfection after natural infection has been documented, but it is not clear how rare this is nor how infectious those with reinfections are.
Until we have more information about the potential to spread disease after infection or vaccination, those who are considered immune should still adhere to public health and social measures designed to reduce the spread of COVID-19. This is particularly important when interacting with people who are not protected against COVID-19. As it may not be clear who is considered immune and because it is not assured that those considered immune are fully protected, everyone—including those who have been vaccinated—should continue to practice the 3 W’s: Wear a mask, Watch your distance, and Wash your hands.
Other characteristics of effective certificates
Any certificate must be a trustworthy and precise vehicle for the information contained. A vaccination certificate, for example, must instill trust that the person presenting the certificate is the person named on the certificate, that they actually received the claimed vaccination, that the vaccination was approved by an appropriate agency, that the vaccine was not counterfeit, that the vaccine was properly stored and administered, that it was administered on the date(s) claimed, and that the person is still adequately protected. Trust in certificates attesting to COVID-19 vaccination, prior infection or immunity will be undermined unless there are standards for each of these factors.
The International Certificate of Vaccination or Prophylaxis (ICVP)—issued in the form of a yellow card with vaccination information—provides a standardized template for tracking an individual’s yellow fever vaccination, as well as treatments or vaccines for other diseases. The ICVP includes identifying information about the holder (name, date of birth, sex, nationality, national ID number if applicable, and signature), notations to support the validity of the document (signature of authorized health worker, official stamp of administering center) and information about the administered vaccine or prophalaxis (manufacturer and batch number for each, date administered, valid start date and end date for protection). Yellow fever, for example, requires a single-dose vaccine that provides protection beginning 10 days after administration and lasts for life for most travelers, though booster vaccine doses may be requested after 10 years when traveling to certain places. The ICVP is only valid for vaccines or prophylaxes that have been approved by the World Health Organization (WHO).

There are several advantages to the ICVP model. It is a widely adopted, inexpensive document that can easily incorporate any COVID-19 vaccines approved by WHO. Because the personal information is held by the certificate holder on a single paper form, it avoids privacy and data security concerns. Complexities include ease of forgery and difficulty of verification, restraints on innovation because of the need for WHO approval before modifying forms, and lack of digital backup if it is lost, stolen or destroyed.
A certificate does not necessarily need to be paper-based. Mobile phone apps, USB drives, bracelets or other innovations can serve the same function. Digital certificates can solve problems of verification and backup but create new challenges of data security, privacy standards and equitable access to technology.
The procedure for issuing digital or paper certificates should be secure. The “My Covid Pass,” tool, developed by the African Union and Africa CDC, provides an example of a system that uses multiple layered security procedures to verify public health documentation and can be used by travellers crossing international borders.

Certain populations might resist certificates if there is a perception that their personal data will be mined by hostile governments, corporations or even hackers. Some U.S. states may refuse to provide CDC with the personal information of vaccinated people, including names, birth dates, ethnicities and addresses. In general, collection of personal data should follow international principles, codified in Article 5 of the E.U.’s General Data Protection Regulation. These principles include (1) lawfulness, fairness and transparency, (2) purpose limitation, (3) data minimization, (4) accuracy, (5) storage limitation, (6) integrity and confidentiality, and (7) accountability.
Ethical questions
Ethical issues of immunity passports have been debated widely in major scientific journals, including JAMA, JAMA Network, Journal of Infectious Disease, BMJ, The Lancet, Journal of Bioethical Inquiry, and the Bulletin of the World Health Organization.
The most searing critiques involve questions of inequity and incentive. The equity critique concerns the limited supply and cost of vaccinations and testing. Vaccines will not be available to everyone immediately and, in many places, access to PCR and antibody tests has been limited. People with the means to access these services will be able to benefit from immunity passports whereas others will not. This can amplify existing inequities where those with more access enjoy the benefits of both health protection from immunity and the social and economic benefits associated with immunity passports, while the rest are unprotected and left behind. This might also undermine any “we are all in this together” messaging.
When immunity passports are contingent on a vaccine, this creates an incentive to get vaccinated, which is a social good. However, when they depend on any form of immunity, this may create a perverse incentive for people to get infected intentionally, especially if they perceive a low personal risk from illness compared to the economic and social benefits of the passport. This concern becomes less likely if everyone has equitable access to the vaccine. On the other side of the debate, some argue that it is unethical to impose the crushing economic and social damage of public health and social measures without a compelling interest. There is a strong individual and societal benefit to releasing people from strict adherence to these measures as soon as it is safe, regardless of how they became immune. These ethical issues can be minimized by increasing the production of vaccines and prioritizing universal, free, and rapid access to them.
Conclusion
We may be able to facilitate reopening safely and equitably using a thoughtful system of risk-conscious licenses—permits from an authority to engage in a particular activity—based on transparent and trustworthy certificates that attest to a person’s immunity based on vaccination or previous infection. Together, these licenses and certificates can serve as immunity passports that can give people access to more places and activities and allow economic and social activities to gradually resume. For example, people who have completed a vaccination series, and, potentially, those who have recovered from a documented SARS-CoV-2 infection within six months may be issued certificates which enable them, while still adhering to mask, distance, and other restrictions, to travel more freely or participate in a wider range of in-person activities. Further study is needed to answer scientific questions about correlations between infection and immunity, immunity and protection, and the longevity of immunity after vaccination. Until we know more about immunity to and protection from COVID-19, any license, certification or immunity passport program should be explicit about what is being attested to and should avoid guarantees of protection against COVID-19. Policymakers should take steps to ensure that any immunity passport program reduces inequities, promotes the health of individuals and communities and does not undermine the practice of critical public health and social measures that are proven to reduce the spread of COVID-19.
FAQ - What do the changes in the CDC’s quarantine recommendations mean for me?
CDC states that while the 14-day quarantine remains their recommendation and has the lowest risk of ongoing transmission, acceptable alternatives include:
- Quarantining for seven days after COVID-19 exposure with daily symptom monitoring. Quarantine can end at seven days with a negative PCR test result (from 24-48 hours before) and no reported symptoms.
- Quarantining for 10 days after COVID-19 exposure with daily symptom monitoring. Quarantine can end assuming no reported symptoms.
Those who exit quarantine after 7 or 10 days should continue to self-monitor for symptoms for the entire 14-day period after exposure. If symptoms develop, they should self-isolate immediately. In addition, they should wear masks and practice other public health and social measures during the remainder of the 14-day window. The seven-day option with testing should only be used if there is sufficient diagnostic capacity. In the case of limited capacity, testing for people with symptoms should be prioritized.
The new guidance from the CDC is an attempt to balance the optimal quarantine time of 14 days with the burden that being in quarantine places on individuals and communities. In addition, if a longer quarantine leads to lower compliance, it won’t reduce transmission overall.
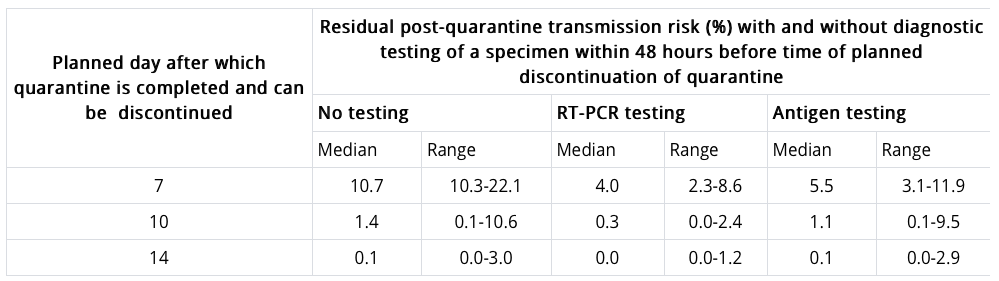
To identify the other acceptable options, the CDC modeled the potential of transmitting COVID-19 after a symptom-free quarantine depending on the length of quarantine and whether a negative PCR or antigen test result was received. Results are in the table below. Notably, the risk of transmitting COVID-19 after a 14-day quarantine is 0.1%; for a 10-day quarantine it’s 1.4%; and for a 7-day quarantine with a negative PCR test, it’s 4% (5% for an antigen test).
Although CDC’s recommendations on quarantine are influential, ultimately, quarantine rules are made by states and localities.
Weekly Research Highlights
A Meta-analysis on the Role of Children in SARS-CoV-2 in Household Transmission Clusters
(Clinical Infectious Diseases, December 2020)
- The study included 57 studies that examined transmission among household clusters (e.g., two or more cases among cohabiting individuals within a two week period), and covered 12 countries. Only 11 studies were included in the analysis of secondary attack rates.
- The authors conducted a number of sensitivity analyses to account for limitations of the study but these did not substantially alter their conclusions. Sensitivity analyses included:
- Assuming that all asymptomatic children were actually the index case (children may be less likely to have symptoms and therefore may not be identified as the index case); in this analysis, 19% of index cases were children.
- Excluding clusters where the index case was due to travel; in this analysis, 21% of index cases were children.
- Excluding clusters that happened during lockdowns, as children were often more affected by lockdowns; in this analysis, 3% of index cases were children.
- Given the hypothesis that children were less likely to have symptoms, the authors examined rates of onward transmission from asymptomatic versus symptomatic index cases. Interestingly, asymptomatic cases were 83% less likely to transmit (RR 0.17 95% CI: 0.09 – 0.29).
- In their analysis of secondary transmission within clusters, there was no significant difference between younger and older children; however, that may have been due to a lack of power: although not statistically significant, younger children had a 31% lower risk of COVID-19 compared to older children.
- Limitations of the study include the fact that the number of household clusters ultimately included was not that large (213). Further, the study does not apply to transmission among children or children and adults outside of the home setting.
Implementing Mitigation Strategies in Early Care and Education Settings for Prevention of SARS-CoV-2 Transmission — Eight States, September–October 2020
(MMWR, December 2020)
- Researchers at the U.S. Centers for Disease Control and Prevention (CDC) collaborated with the Administration for Children and Families to conduct mixed-method qualitative evaluation of Head Start and Early Head Start programs in eight states. These are federally funded programs that provide early learning and healthy development programs for children 0-5 years old, as well as programs for pregnant women.
- Although all of these centers closed for two to eight weeks in April and May, they reopened with mitigation strategies in place as recommended by CDC. Few cases of COVID-19 were reported during May and June (nine cases total from three of 55 centers responding to the survey study). Interviews conducted in September and October to better understand how mitigation strategies were implemented revealed that program administrators relied on extensive communication and consistent messaging to ensure the safety of program attendees and staff.
- Common strategies implemented were: reinforcement of hand hygiene behavior and respiratory etiquette, supervised hand-washing and hand-sanitizing for children, intensified cleaning and disinfection efforts (e.g., with toys, frequently touched surfaces, and bedding), required use of masks for staff members, visitors and children aged >2 years, physical distancing to the extent possible, daily health screening procedures on arrival for children and staff members, drop-off and pick-up procedures, monitoring for absenteeism, steps to increase ventilation, including installation of ion air purifiers, steps to decrease occupancy in areas without increased ventilation, use of outdoor space as much as possible, and cohorting by classroom to minimize exposure between groups.
- The findings in this qualitative study are subject to some limitations, including full attribution of low case counts to implementation of mitigation strategies. However, given documentation of transmission of SARS-CoV-2 in these settings and previous evidence supporting the use of various mitigation measures, the authors support using these measures to help keep child care and early education programs open. They note that additional information is needed for areas with high community transmission.
Suggested citation: Cash-Goldwasser S, Kardooni S, Cobb L, Bochner A, Bradford E and Shahpar C. In-Depth COVID-19 Science Review December 5 – 11, 2020. Resolve to Save Lives. 2020 December 15. Available from https://preventepidemics.org/covid19/science/review/








