There will be no Weekly Science Review next week. We will resume on September 15th.
In depth
COVID-19 reinfection
The Hong Kong case
On August 24, researchers in Hong Kong announced the first confirmed instance of human reinfection with SARS-CoV-2. The manuscript detailing the supporting scientific evidence provides the following details: a 33-year-old otherwise healthy man living in Hong Kong developed three days of respiratory symptoms and was diagnosed with COVID-19 on March 26. After recovering, he was subsequently tested twice more for SARS-CoV-2, in accordance with local policies around ending COVID-19 patient isolation, and both tests were negative. On Aug. 15 he was tested for SARS-CoV-2 again as part of reentry screening when he returned to Hong Kong after a trip to Europe. That test result was positive. He had no symptoms at the time of the second positive result. Genome sequencing revealed that the viruses isolated from throat swabs obtained in March and in August were from different genetic groups, leading the study authors to conclude the patient had been infected twice.
What makes this report of reinfection different from previous reports?
There have been numerous reports of patients with possible reinfection prior to this one. In April, it was reported that hundreds of people in South Korea who had recovered from COVID-19 and were retested for SARS-CoV-2—as part of a government screening program or after exhibiting new symptoms—had tested positive upon retesting. In all of these cases of possible reinfection, initial and subsequent tests were polymerase chain reaction (PCR)-based. Such tests, which are recommended to diagnose active infection with SARS-CoV-2, detect the presence of SARS-CoV-2 genetic material but cannot determine whether the virus is alive or how long it has been present, or fully describe its genetic composition. To help determine whether the patients in South Korea had indeed been reinfected and if they could transmit the virus, researchers attempted to culture SARS-CoV-2 from retest samples and traced the close contacts of those with positive retest results. Virus could not be cultured and there were no confirmed COVID-19 cases among contacts, suggesting that the detected virus was not alive. It is therefore thought that what was observed in South Korea was prolonged shedding of SARS-CoV-2, a phenomenon that is now well described. Studies have also shown that in patients with prolonged viral shedding, PCR testing may alternately yield negative and positive results, and these patients do not appear to be infectious to others.
Even if live virus had been cultured from those who retested positive for SARS-CoV-2 or if transmission months after initial diagnoses had been observed in South Korea, that alone would not have distinguished between prolonged viral shedding and reinfection. The best way to establish proof of reinfection is to perform genome sequencing, as was done for the case in Hong Kong. Genome sequencing refers to the process of determining the order of chemical building blocks that comprise the genetic code of an organism. Although the genomes of different SARS-CoV-2 virions (individual virus particles) are very similar to each other—hence they are all identified as SARS-CoV-2 and not as other viruses—differences do occur. Those differences develop through mutations, or the substitution of one chemical building block for another, as genome copies are made. Mutations may be inherited by the next generation of virions, resulting in viral evolution as they accumulate over time. Genome sequencing can thus help determine whether two populations of SARS-CoV-2 evolved separately from each other or whether one gave rise to the other; this principle can be applied to virus samples obtained from a single person at two different times. Thousands of genome sequences of SARS-CoV-2 isolates from all over the world have been published in online databases; comparison and analysis of these sequences has resulted in the characterization of several clades. A clade is a group of organisms that can be traced to a common ancestor and all common descendants. Clades of SARS-CoV-2 have geographic specificity in part because viral evolution has occurred after SARS-CoV-2 has been transported between continents. For example, viruses from one lineage, clade G, predominate in Europe and the United States, while clade L, the progenitor of clade G, predominates in Asia. In the case of the Hong Kong patient, genomic sequencing determined that the patient’s first infection was caused by virus from clade V, while the second was caused by virus from clade G. This strongly suggests that the patient was infected on two separate occasions, in different parts of the world.
After the announcement from Hong Kong, a preprint article detailing a case of reinfection in the United States was published online. In that case, a 25-year-old otherwise healthy man living in Nevada developed symptoms consistent with respiratory viral infection and was diagnosed with COVID-19 on April 18. The patient recovered and two tests for SARS-CoV-2 performed in May returned negative results. At the end of May, the patient again developed symptoms consistent with respiratory viral infection, and he tested positive for SARS-CoV-2 in early June. During this second episode of illness, he required hospitalization and oxygen therapy. Genomic sequencing revealed that both viral isolates were from clade C but also that there were a number of genetic differences between the isolates. Based on the rate of mutations typically observed for SARS-CoV-2 and the degrees of genetic differences between the two isolates, authors concluded that it is “virtually assure[d]” that these were two distinct infections. There have also been recent news reports of one case of reinfection in Belgium and one in the Netherlands diagnosed using genome sequencing, but the scientific details of those cases were not available as of this writing.
Is the occurrence of reinfection surprising?
The possibility of reinfection with SARS-CoV-2 has been widely debated. Several lines of evidence have been used to argue against it. A study conducted early in the pandemic showed that rhesus macaques who cleared a first infection with SARS-CoV-2 and were reexposed to the virus did not get infected a second time. Infection with other betacoronaviruses that cause SARS and MERS appears to result in long-lasting immune responses which may be protective. Until the announcement from Hong Kong, evidence to support the occurrence of reinfection had not been published. On the other hand, the occurrence of SARS-CoV-2 reinfection is not surprising based on experience with other infectious diseases. “Sterilizing immunity,” or complete protection from infection after immunologic priming by natural infection or vaccination, is often an elusive target, either because short-term immunity is not foolproof or because immunity tends to wane over time. There are examples of infectious diseases, including the endemic human coronaviruses that cause the common cold, which may cause multiple reinfections within a relatively short period of time. Evidence from animal studies demonstrates the possibility of SARS-CoV-2 infection despite immunologic priming: during preclinical studies on the candidate COVID-19 vaccine from Oxford, rhesus macaques became infected with SARS-CoV-2 when exposed after vaccination, evidenced by the recovery of viral genetic material from their noses.
If reinfection is possible, why are the first cases of this only being reported now? Will there be more cases in the future?
It is possible there have been other cases of reinfection that have not been detected or convincingly investigated. Some studies on prolonged shedding have not performed genomic sequencing to investigate whether a new infection may have been present. If second episodes are more likely to be asymptomatic, diagnosis of reinfection may require detection of asymptomatic infection in addition to comparison of genetic sequences, including of virus from samples that may have already been discarded. In essence, public health surveillance systems are not set up to identify cases of reinfection. Based on current information, it is difficult to predict how commonly reinfection will occur, but it is useful to explore whether features of the Hong Kong and Nevada cases make those patients’ situations more or less applicable to other patients. Aspects to consider include the immune response of the patients to SARS-CoV-2, the timing of reinfection, and the relatedness of the two infecting viral strains.
Immune response of the patients. The patient in Hong Kong was tested three times for IgG antibodies against SARS-CoV-2: 10 days after his first infection was diagnosed, and one and five days after his second infection was diagnosed. The first two results were negative and the third result was positive. It is unclear what to make of these data. It is possible that the patient did not mount a detectable IgG antibody response after his first infection; similar findings have been documented in up to 8% of patients in other studies. It can take an average of two weeks after symptoms begin for IgG antibodies to appear. Thus, it is possible that the patient’s first test was conducted too soon; the detection of IgG antibodies only five days after the diagnosis of reinfection indicates there may have been an antibody response to the first infection that was initially undetected and/or was boosted by reinfection. Whether or not the patient produced IgG antibodies after his first infection, antibodies are just one marker of the immune response to SARS-CoV-2, and the correlation between antibodies and immunologic protection is unclear. Indeed, some people who have been exposed to SARS-CoV-2 generate strong immune responses despite the absence of detectable antibodies. Thus, it is not possible to say what the Hong Kong patient’s level of immunologic protection was or whether it was unusual; it is only possible to say that failure to mount a detectable antibody response 10 days after initial infection has been described in other patients. In the case of the Nevada patient, he was tested for antibodies to SARS-CoV-2 soon after his second diagnosis in early June. The IgG antibody test result was positive at this time; those antibodies may have developed after the first or second infection.
Timing of reinfection. It is unclear how much the time period between infections—4.5 months in the case of the Hong Kong patient, and six weeks in the case of the Nevada patient—may have contributed to the patients’ risks for reinfection. Studies have shown that SARS-CoV-2 antibodies can decline rapidly within a few months of infection but again, the role that antibodies play in immunologic protection is unclear. It is possible these patients were poorly protected against reinfection and were reinfected as soon as they were next exposed to SARS-CoV-2. Generally speaking, immunologic protection induced by natural infection or vaccination tends to wane over time, hence the possibility that an effective COVID-19 vaccine may need to be administered repeatedly in order to maintain sufficient immunity. It is possible that reinfection will occur more frequently as increasing time elapses after more people recover from their first COVID-19 infections.
Reinfection with different strains. Immunologic protection is most effective when the immune system can easily recognize a pathogen. Indeed, a different influenza vaccine formulation must be produced each year because the influenza virus mutates so rapidly. A pathogen can evade immune recognition after immunologic priming if mutations are present in the genes that code for the viral proteins to which the immune system responds. For SARS-CoV-2, one of these proteins is the spike, or “S” protein. Specific to the Hong Kong case, viruses in clades G and V do display differences in the genes that encode their S proteins, and genomic sequencing of the two viral isolates from the patient confirmed differences in their S protein genes. It is unknown whether those differences overcame an immune response that would have prevented reinfection if the second strain of SARS-CoV-2 was more similar to the first. It is possible that as travel restrictions ease and population movement increases, exposure to SARS-CoV-2 clades that have not been previously encountered may increase the risk of reinfection. In the case of the Nevada patient, it is unclear if the observed mutational differences between the two viral isolates may have affected the ability of the immune system to fight off the second infection.
What are the implications of these reported cases of reinfection?
In terms of the clinical implications of reinfection, for other infectious diseases, it is common to observe symptoms of reduced severity when infection occurs after immunologic priming by natural infection or vaccination. Indeed, vaccines which do not offer complete protection against infection can still have a major impact on the burden of disease by mitigating disease severity. One example of this is the influenza vaccine: some people get the flu even after they have been vaccinated, but vaccination can reduce the risk of hospitalization, admission to the intensive care unit, and death. Among the macaques in the Oxford vaccine study who were infected with SARS-CoV-2 despite vaccination, the vaccine appeared to moderate the course of infection: SARS-CoV-2 did not spread to the macaques’ lungs and the macaques did not develop symptoms. Thus, the Hong Kong patient’s lack of symptoms at the time of his second diagnosis may be expected. However, the Nevada patient had more severe disease at the time of his second diagnosis. There are examples of infectious diseases, such as dengue virus, that can cause more severe symptoms upon reinfection, in a phenomenon known as “immune enhancement.” Concerns have been raised about whether immune enhancement may occur for SARS-CoV-2 and, in particular, if vaccination against COVID-19 may precipitate severe disease if post-vaccination infection occurs. At this time, there is no evidence from human or animal studies that SARS-CoV-2 infection can precipitate immune enhancement of disease. It is also possible that what occurred in the Nevada case was not immune enhancement so much as it was what is frequently observed in clinical medicine: if recovery from a first illness has been incomplete or it was a mild illness that didn’t result in a strong immune response, more severe disease can result from a subsequent infection. Alternatively, other host or viral factors could have explained the greater severity of the second infection. Regardless, two cases of reinfection cannot be used to draw conclusions about what is likely to be observed in other cases of reinfection.
The broader public health implications of these cases are also unclear. Importantly, at this point, reinfection appears to be a relatively rare occurrence. Even if some cases of reinfection have been missed, there have been over 24 million reported cases of COVID-19 in comparison with two convincingly demonstrated cases of reinfection. Reinfected patients may be less likely to transmit the virus than patients with primary infections. If there is greater immunologic control of the virus and the amount of live virus is reduced, spread may be less likely, as has been observed after vaccination against other viruses. Lastly, the possibility of infection after immunologic priming may not drastically change approaches to vaccinating the population when an effective COVID-19 vaccine becomes available. A major goal of vaccination is to induce a level of herd immunity such that the transmission rate falls, without the expectation that transmission will fall to zero. Especially if reinfection is relatively rare and reinfected people transmit the virus less frequently, the possibility of reinfection may not significantly change vaccination coverage targets. Additionally, as noted above, a number of vaccines significantly reduce illness and death even if infection is not always prevented. This may be particularly significant in the context of COVID-19, if a vaccine could reduce the proportion of infections that cause significant illness and death.
FAQ
Is it safe to fly during the COVID-19 pandemic?
Travel can increase the risk of contracting or transmitting COVID-19. As many of the strict restrictions and outright bans imposed early in the pandemic are lifted, many people are contemplating travel again. Air travel can seem like a particularly risky proposition; being confined for hours at a time in an enclosed space with hundreds of strangers sounds precisely like the sort of setting that global health experts recommend we avoid. Perhaps surprisingly, there have been relatively few documented instances where COVID-19 transmission has occurred in flight, although that can be difficult to prove. In one superspreader event, a single infected person apparently infected 13 other passengers and crew on a long-haul flight from London to Hanoi. In another instance, 12 symptomatic COVID-19 infected travelers boarded a trans-Pacific flight and no secondary cases were confirmed in a follow-up investigation of 328 other passengers. From January to March, only three instances of suspected in-flight transmission had been reported to the International Air Transport Association. At the time, testing capability was limited and the risk of COVID-19 being transmitted by people without symptoms wasn’t widely appreciated.
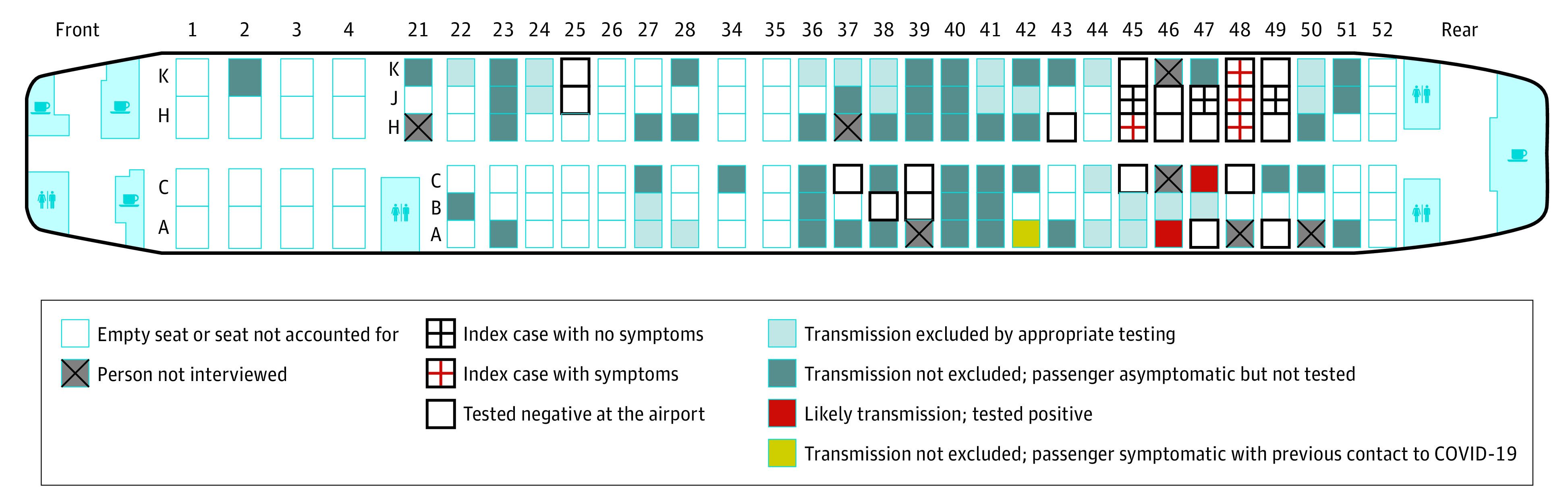
Since then, additional reports have convincingly described apparent transmission occurring between passengers, among cabin crew, and between the two. One of the best characterized occurrences was published in JAMA in August. Twenty-four members of an organized tour group flew from Tel Aviv to Frankfurt seven days after having had contact with a hotel manager who later developed COVID-19. None of the tour group passengers were ill and mask use was not recommended on the aircraft at the time. On arrival, the tour group members were tested for COVID-19 and seven were found positive. Through follow-up symptom surveys and COVID-19 testing, two apparent secondary cases that may have been acquired during the five-hour flight were detected among the remaining passengers. These passengers had been seated near the section of the plane occupied by the seven index cases traveling with the tour group. Neither had contact with a COVID-19 case patient before the flight. One additional passenger had an equivocal test result but also an earlier potential contact where they may have been infected. No cases were detected in crew members or in passengers who had been seated in other parts of the plane.
Figure: Seating chart for a Boeing 737-900 flight with seating location of tour group members exposed to COVID-19 seven days earlier, including seven index cases and two probable secondary cases that may have been acquired in flight.
It is almost certain that other cases have been acquired in-flight, but not identified. Even so, there are also reasons why the airline cabin may not be as risky for COVID-19 transmission as it might first appear. To begin with, industry standards devised and implemented years ago to prevent other respiratory infections almost certainly reduce the likelihood of SARS-CoV-2 transmission. Precision air filters and improved ventilation (described in our last Weekly Science Review) are already implemented on commercial flights. As more air travel providers require face masks, the risk of acquiring COVID-19 while aloft may be further reduced, but will not be eliminated entirely. Air travel also requires spending time in transit to and from the airport, in security and immigration queues, and in waiting and service areas frequented by many others. All of these bring travelers into close contact with one another and with high contact surfaces that may be even riskier than the interior cabin of the aircraft.
Staying home is still the safest way to prevent COVID-19, and travel will always involve some increased risk that has to be carefully weighed. The U.S. Centers for Disease Control and Prevention (CDC) advises potential travelers to consider several issues thoroughly before deciding to leave home, including: how much COVID-19 transmission is occurring at their destination, whether they or someone they live with is at increased risk for severe COVID-19 illness, and whether the destination is enforcing requirements or restrictions for arriving travelers. CDC’s online resources can help identify information about U.S. and global travel destinations. Don’t travel if you are ill or have been around someone with COVID-19 in the previous 14 days. Don’t travel with someone who is ill. If you do travel, make sure to protect yourself and others by wearing a mask, watching your distance, and washing your hands or using an alcohol-based hand rub frequently.
What is convalescent plasma?
Plasma is a thick yellow liquid that is left over once red blood cells are removed from blood. It contains many of the other active components of blood, such as clotting factors and antibodies, and has various applications in medical therapy. Convalescent plasma refers to plasma that has been donated by someone recently recovered from illness. It can play a specific role in treating certain infections because it contains disease-fighting antibodies developed by the immune system that helped the donor recover, and may boost the recipient’s ability to fight the same type of infection. Convalescent plasma as a therapy for infection does not work the same way for all diseases. Although it has been used for more than 100 years, its applications remain limited. With novel and emerging infections, however, it is often studied early as a potential therapy while researchers work on other strategies to prevent ongoing infection and definitively cure disease. Its use has been studied as a treatment for infection from Ebola, H1N1 influenza, severe acute respiratory syndrome (SARS), and Middle East respiratory syndrome (MERS) with mixed results. Like any transfusion, administering convalescent plasma has risks, including allergic reactions, transfusion-related reactions, heart and lung problems, and, rarely, a bloodborne infection. All donations in the U.S. are screened for blood type and infections such as hepatitis B and C, HIV, and others to minimize these risks.
Scientists in China were the first to study convalescent plasma as a possible treatment for severe illness from COVID-19. These early studies were small case series or observational studies that cannot produce definitive results about the research-based efficacy or real-word effectiveness of any treatment. Convalescent plasma has since been under study globally, including in the U.S., with mixed results. Although studies are underway, we do not currently have results from randomized controlled trials for the efficacy of convalescent plasma in treating COVID-19, the gold standard for determining whether or not a therapy works.
Until recently, convalescent plasma could only be used in very specific settings in the U.S. such as in a research study. On August 23, the U.S. Food and Drug Administration (FDA), the regulatory body responsible for approving new therapies for general use, issued an emergency use authorization for the use of convalescent plasma in treating COVID-19. The FDA stated that it believed the totality of the best evidence currently available suggests that convalescent plasma may shorten the duration of illness and possibly reduce the severity of illness, and that these potential benefits outweighed the risks of the therapy. The emergency use authorization allows individual providers to decide if convalescent plasma may be an appropriate therapy for a COVID-19 patient outside of the research or compassionate-use setting.
The definitive demonstration of the efficacy, dosage, optimal timing of treatment, optimal content of plasma, and safety of convalescent plasma for treatment of COVID-19 cannot be established without evidence from rigorous randomized controlled trials designed to answer these questions.
What is Abbott’s new 15-minute test for COVID-19?
On August 26, 2020, the U.S. Food and Drug Administration issued an emergency use authorization for Abbott Laboratories’ new COVID-19 test, BinaxNOW. The test is an antigen test which was described in a previous Weekly Science Review here, and will not need to be processed in a laboratory or use any specialized machine to give results. This type of antigen test does not test for the complete virus itself or its genetic material. Rather, it reacts with antigen proteins on the virus’ surface in actively infected people to produce a positive result, or negative result if no antigens are detected. It is self-contained and can be administered at the point of care, such as at a provider’s office, on-site in nursing homes, or in a school’s health office. The test uses similar technology to rapid flu and rapid strep tests called lateral flow assays.
BinaxNow will give results in 15 minutes and will sell for $5, making it the cheapest and fastest test currently on the market. Given recent supply shortages and delays in testing, its broad availability may help significantly increase testing capacity in the country, although it will still require a nasal swab to collect a sample. It will also be linked to a free phone-based app called NAVICA which will allow people to display their results to an interested party. Its performance has been studied mostly in people who presented to a health care provider within seven days of developing symptoms compatible with COVID-19. It performed well in this setting, but far less is known about how it will perform in people who do not have symptoms. This is being studied further. The FDA states on its website that antigen tests are more likely to miss infections compared to molecular tests, and that some people may need a molecular test to confirm the result of the antigen test depending on a provider’s level of concern.
Weekly Research Highlights
BCG Vaccination in Infancy Does Not Protect Against COVID-19. Evidence from a Natural Experiment in Sweden
(Clinical Infectious Diseases, Aug. 23)
- Early in the COVID-19 pandemic, researchers noted that countries where universal vaccination with BCG has been implemented in infancy or childhood may have experienced fewer cases of COVID-19 or a milder spectrum of illness than countries where BCG was not recommended. Several ecological studies produced conflicting results. Making comparisons across countries in this way makes it impossible to know whether BCG vaccination or some other factor, either known or unknown, actually caused the observed differences.
- In Sweden, universal BCG vaccination at birth was recommended until April 1975. Before this change, nearly all children (92%) received BCG in infancy compared to very few (2%) who were born later. Researchers used publicly available datasets to compare the risk of COVID-19 illness, hospitalization and death in May 2020 among successive cohorts of people born before and after the BCG policy change.
- In a regression discontinuity analysis Swedish adults born just before and just after April 1975 had similar risk of developing COVID-19 illness and of being hospitalized for COVID-19 45 years later. The authors conclude that BCG vaccination at birth did not protect against COVID-19 in middle-aged adults. The large study size and the ability to compare groups with different BCG exposure in the same country are more convincing—at least for the environment in Sweden—than findings from some earlier ecological studies and may be the best source of evidence possible to detect any effect of BCG at birth might have on COVID-19 risk in adults today.
This study does not provide any information about whether giving BCG vaccine now could be an effective treatment or preventive measure for COVID-19. Several ongoing or planned clinical trials aim to evaluate what impact, if any, recent BCG vaccination can have on infection or clinical outcomes, but the first results won’t be available for months.
Suggested citation: Cash-Goldwasser S, Kardooni S, Cobb L, Bochner A, Bradford E and Shahpar C. In-Depth COVID-19 Science Review August 22 – 28, 2020. Resolve to Save Lives. 2020 September 1. Available from https://preventepidemics.org/covid19/science/review/