Opening K-12 schools for in-person learning amidst vaccines and variants: what have we learned and how can this guide us?
Epidemiology and Presentation of SARS-COV-2 in Children
From March-December 2020, a CDC analysis found that COVID-19 case rates were consistently lowest among children age 0–10 years. Incidence among adolescents was higher than among children, but lower than among adults. In general, trends in cases among children and adolescents (under age 18) paralleled those among adults, including during months when some U.S. schools were open for in-person education. In contrast, incidence among young adults (age 18–24 years) was higher than that in other age groups throughout the summer and fall, with peaks in July and September that preceded increases among other age groups, suggesting that young adults might contribute more to community transmission than children.
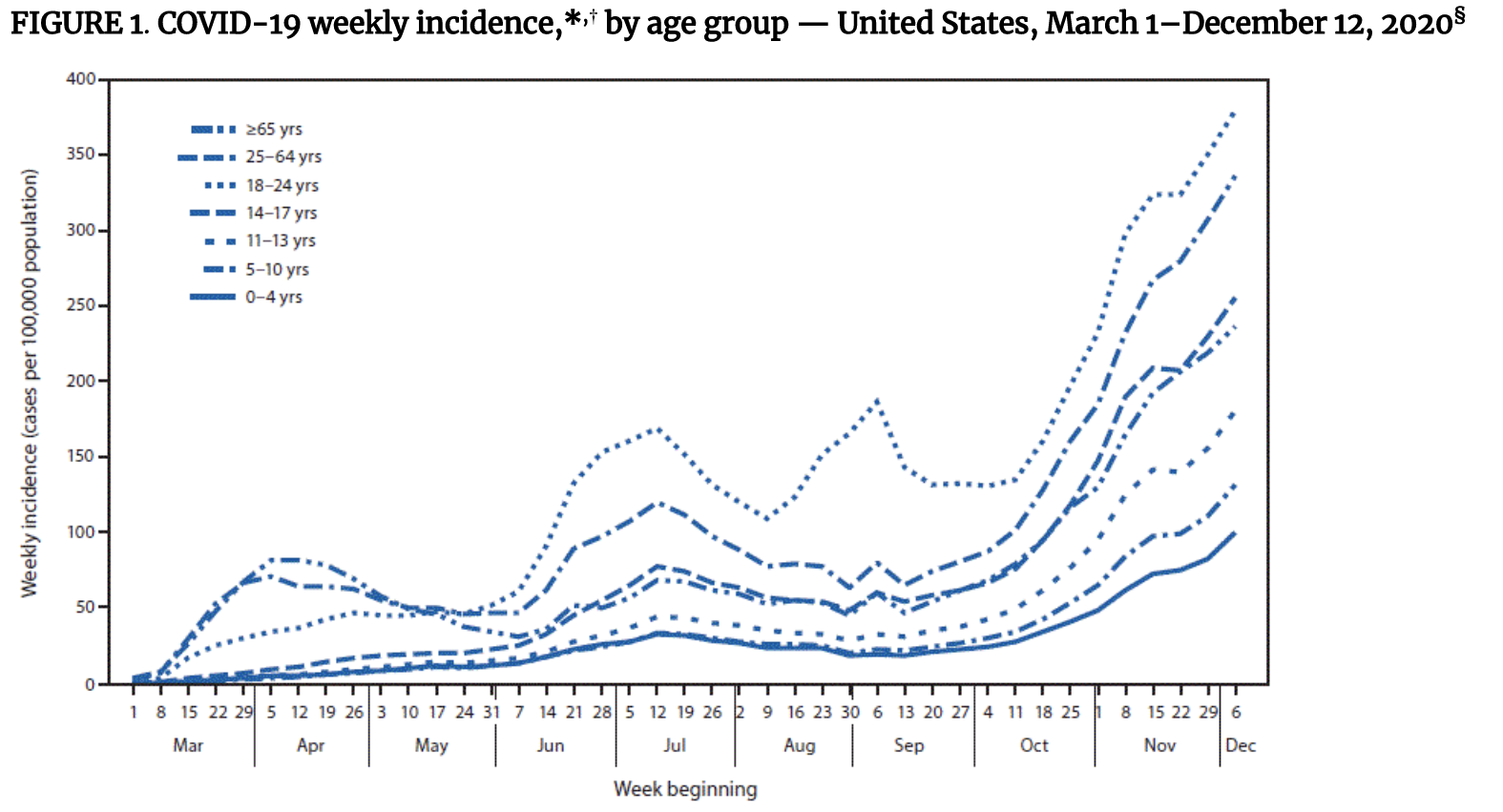
Source: CDC
These trends suggest that COVID-19 incidence among children may be lower than among adults, that transmission among children does not substantially drive transmission in older age groups, and that school reopening does not contribute to increased transmission among older age groups. However, because younger children may have fewer exposures to COVID-19 compared to adolescents and adults and, because they are much less likely to become severely ill, may be less likely to be tested, analyses of population-level case data can obscure the extent and direction of transmission among different age groups. To better understand transmission risk and patterns in different age groups, analyses of different types of data are needed.
Are children at similar risk of getting COVID-19 as adults?
Younger people, and particularly children under 10 years of age, may have lower susceptibility to SARS-CoV-2 infection than adults. Two types of studies lend support to this finding: household contact tracing and seroprevalence studies. One way to determine the difference in susceptibility to infection between children and adults is to examine the extent to which transmission occurs in environments where both children and adults are exposed to SARS-CoV-2. Household contact tracing data can be used for this purpose. Such studies often estimate a secondary attack rate, which is the proportion of people who are infected (secondary cases) among all people exposed to the person identified as the first case (index case) within the household. A systematic review on SARS-CoV-2 transmission patterns analyzed data from 14 studies on household transmission and found that adult contacts of index cases were more likely to be infected than contacts under 18 years of age: the secondary attack rate among adults was 33%, compared with 17% in children. Another systematic review, on household transmission of SARS-CoV-2, reported similar household secondary attack rates: on average, 28% of adult contacts compared to 17% of child contacts became infected.
Several factors may artificially reduce the observed secondary attack rate among children. Infections may not be detected among children if they are asymptomatic or have mild symptoms, or if symptoms in children – especially in the youngest age groups – are difficult for their caregivers to detect. However, a systematic review that attempted to address this bias by focusing on studies in which all contacts were universally tested regardless of symptom status also found higher secondary attack rates in adult contacts than in child contacts. Another potential source of bias is that higher secondary attack rates in adults may reflect different exposure patterns among adults (i.e., more close contact with the index case) versus children within the same household. This is supported by studies which suggest that the secondary attack rate among spouses of index cases is higher than among non-spousal adult household contacts.
Another source of data on susceptibility among children versus adults is seroprevalence data, or data on the proportion of the population that has antibodies to SARS-CoV-2 as determined by a blood test, suggesting that an infection has previously occurred. Some seroprevalence studies have estimated that young adults and adolescents have higher seroprevalence than older age groups. However, seroprevalence surveys often do not represent the general population (e.g., they use samples obtained from blood donors). One study that reviewed seroprevalence studies that were broadly representative of a nation, region or city and reported age-specific data found that most reported lower seroprevalence among those 20 years of age or younger compared with adults (over age 20), although seroprevalence among those over 10 years of age was more similar to seroprevalence among adults.
Together, data from household and seroprevalence studies suggest that children may be less likely to become infected with SARS-CoV-2 compared to adults. As discussed below, whether the emergence of new, more highly transmissible variants will change this pattern is not yet known; evidence suggests that the increased transmissibility associated with some variants applies across age groups; thus, the relative difference in susceptibility between adults and children might remain the same.
What happens when children do get COVID-19?
1. Acute disease
Most children infected with SARS-CoV-2 are asymptomatic or experience mild illness. A systematic review of 131 studies from 26 countries found that the most commonly reported symptoms were fever (reported by 59% of children) and cough (55%), whereas an estimated 19% of infected children were asymptomatic. However, some studies estimate that a much higher proportion of cases in children may be asymptomatic, including one study of more than 200 children with COVID-19 in Greece which found that 54% of infections in children were asymptomatic. In most settings, testing among children without symptoms may be inadequate to estimate the true proportion of asymptomatic cases. Therefore, it is not clear whether the proportion of infections that are asymptomatic is higher among children than adults.
The relatively low severity of COVID-19 among children contrasts with some other respiratory viruses that can cause particularly severe disease among children, such as respiratory syncytial virus and influenza. However, lower prevalence of severe disease among children has been observed for both SARS and MERS, which are caused by coronaviruses that are related to SARS-CoV-2. Research is ongoing to explore hypotheses that might explain why COVID-19 is less severe among children. For example, children may have a decreased number of ACE-2 receptors, the part of the cell used by the virus to gain entry, in their respiratory tracts. It has also been proposed that children have different immune responses than adults, and these responses may be more protective. It may be a combination of factors, including a lower prevalence of other underlying medical conditions, that put children at lower risk for severe COVID-19 illness.
Cases of severe COVID-19 illness, hospitalization and death have occurred among children, although much less frequently than among adults. Early data from the WHO-China Joint Mission on COVID-19 showed that 2.5% of cases among people under 19 years old were severe and 0.2% were critical. Data assembled from 23 U.S. states and New York City by the American Academy of Pediatrics show that children represented 1-3% of total COVID-19 hospitalizations and that less than 2% of all COVID-19 cases in children have resulted in hospitalizations. COVID-19 deaths in children are rare. As of June 30, 2021, CDC reported more than 3.3 million cases and 471 deaths (0.01% of cases) among children under age 18 in the U.S. A recent preprint identified 25 deaths caused by COVID-19 among children under 18 years of age in England during the first year of the pandemic (March 2020-February 2021) whereas 99.995% of infected children survived.
A disproportionate impact of COVID-19 on more vulnerable children has also been documented. Among adolescents (12-17 years) hospitalized with COVID-19 at more than 250 hospitals in 14 U.S. states during March 2020-April 2021, approximately two thirds were Hispanic or non-Hispanic Black persons. This is consistent with abundant evidence on the disproportionate impact of COVID-19 among Black and Hispanic populations. In Brazil, Black, Brown, and Indigenous children hospitalized with COVID-19 also were at higher risk of death compared to White children. The proportion of children who died without ICU care was highest in the poorest regions of Brazil. These findings reflect the potential impact of a range of factors that may increase the risk of worse outcomes among children from racial and ethnic minorities, including lack of access to adequate care, medical comorbidities, increased risk of exposure and the effects of poverty and systemic racism on health.
2. Post-acute sequelae
In addition to the risk associated with acute illness, COVID-19 also may pose longer term risks to children’s health. We previously wrote about ‘long COVID,’ a condition in which COVID-19 patients experience symptoms weeks to months after acute infection. Information about persistent symptoms among children is limited. One study of 129 Italian children found that 55 (43%) experienced one or more symptoms 60 or more days after their initial infection, including some children who were initially asymptomatic. Another post-acute syndrome that affects some children with COVID-19 is multisystem inflammatory syndrome in children (MIS-C), a rare but potentially life-threatening condition. Approximately 300 cases of MIS-C may occur per million SARS-CoV-2 infections in persons younger than 21 years. MIS-C is characterized by inflammation of a variety of organs, including the heart, lungs, brain, kidneys, gastrointestinal system, skin and eyes. The condition may be caused by an aberrant immune response, but our understanding of the pathophysiology of MIS-C is incomplete and we do not know why some children are susceptible to MIS-C whereas others are not. Over 70% of MIS-C cases occur in previously healthy patients. To date in the U.S., more than 4,100 cases and 37 deaths due to MIS-C have been reported to the CDC. Black and Hispanic children make up most (62%) of these MIS-C cases. Additional research is needed to better understand long-term sequelae of COVID-19 among children, including MIS-C, and disparities in which children are affected.
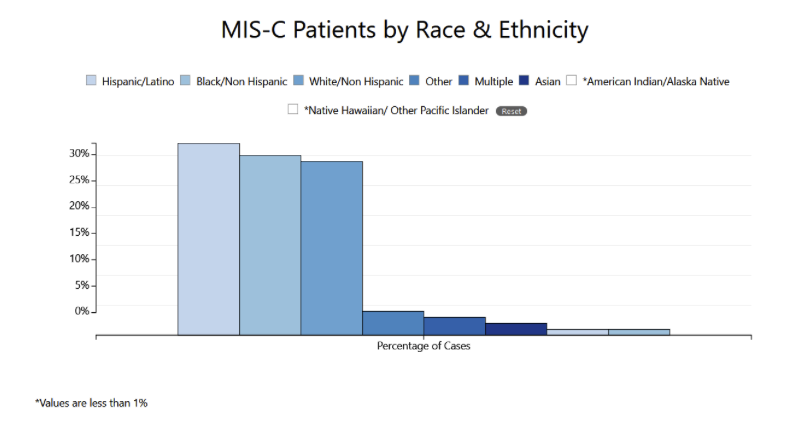
Source: CDC
What happens when children get COVID-19 caused by a new variant?
As new variants of the SARS-CoV-2 virus have emerged, there have been questions about whether children are at greater risk of severe illness. Assessing the severity of new variants is challenging. As recently outlined in the Atlantic, a key challenge is that the virulence, or severity of illness caused by a virus, depends not only on the virus but also the characteristics of the person infected and their environment (e.g., availability of health care). To assess the virulence of new variants, researchers typically look for differences in the frequency of hospitalization or death among persons with COVID-19 infected with different variants. However, as we wrote previously, robust data on COVID-19 hospitalizations and deaths are not available in all countries. In addition, criteria for hospitalization vary depending on healthcare system capacity and clinical practice guidelines, which have changed during the pandemic. Further, the variants of concern that have emerged to date are more transmissible than earlier strains of SARS-CoV-2. The ratio of mild to severe cases could remain the same, indicating no differences in virulence, but a more transmissible variant could infect and sicken more people, leading to a greater number of hospitalizations and deaths. In addition, large waves of cases caused by more transmissible variants may overwhelm the healthcare system, which can lead to increased mortality among patients who might otherwise have survived. Furthermore, differences in severity associated with different variants are even more difficult to establish in places where genomic sequencing capabilities are limited. Increases in vaccination coverage also make it more difficult to compare the relative severity of variants that emerge at different times.
Despite these challenges, available research describing the severity of variants of concern are summarized by WHO in the table below

Source: WHO
Emerging data are teaching us more about potential differences in overall disease severity associated with variants of concern, but there is little evidence about whether these variants may cause more severe illness among specific age groups, including children. To explore whether the alpha variant may cause more severe illness among children, investigators in the U.K. compared the characteristics of patients aged 18 or younger admitted to King’s College Hospital with COVID-19 during two time periods: March-May 2020 and November 2020-January 2021. The alpha variant predominated in the UK during the latter period. The demographic characteristics of patients were similar, few patients required oxygen therapy or ventilation during both waves, and the proportion of patients requiring this kind of treatment was lower during the second wave. Although the number of children admitted to the hospital was higher during the second wave (60 vs. 20 admitted patients), the number of adult patients increased similarly, likely because of higher prevalence of COVID-19 in the community during the second wave. The authors concluded that there was no evidence of more severe disease in children associated with the alpha variant. Data are not yet available to compare severity of disease in children caused by the delta versus other variants. Although the media has reported an increase in infant deaths in Indonesia during the current wave caused by the delta variant, the Indonesian Pediatric Society suggested that the increase may be due to reduced adherence to protective measures and lack of awareness of the risk posed by COVID-19 to children, rather than increased severity of the variant. Similarly, deaths among children under 5 years reported in Brazil at rates far higher than elsewhere in the world may be explained in part by prolonged community transmission at high levels, delayed diagnosis and initiation of treatment, uneven access to care and overloaded hospital systems. It is not clear to what extent the Gamma variant, which has predominated in Brazil for several months, may contribute to severe outcomes.
Transmission and Mitigation Measures in Schools
When transmission occurs in schools, what are the patterns?
The extent to which children versus adults contribute to the overall spread of COVID-19 is not clear, but studies suggest that children may spread infection less than adults within equivalent settings. There is evidence that when the index case in a household is under 20 years of age, there is less transmission within the household. Reduced disease spread from children may help explain some of the age-specific transmission patterns observed in schools.
Data show that within schools, students are less likely than staff to be index cases. After the first national lockdown in England, the government conducted enhanced surveillance from June 1–July 17, 2020 in educational settings that reopened. During that time, there were 55 outbreaks. Of 210 total cases linked to outbreaks, most (73%) were in staff members, and probable direction of transmission was staff to staff in 26 outbreaks. Data from Australian schools from March–August 2020 suggested that children transmitted less than adolescents and adults. Fewer cases in primary schools went on to become outbreaks compared to cases in secondary schools, and if the first case was in a child younger than 5, an outbreak was very unlikely.
Several studies have found that when outbreaks occur in schools, they are generally small. Over 90% of outbreaks that occurred in Australian schools between March–August 2020 involved 10 cases or fewer. Similarly, a European Centre for Disease Prevention and Control report from December 2020 that included findings from 17 country-level surveys found that 12 countries reported clusters of cases that had been linked to schools, but most clusters involved fewer than 10 cases. In addition, clusters often could not be definitively linked to school- versus community-based transmission. This report also concluded that school staff are not at greater risk of COVID-19 than adults working in other professions. For example, an analysis of data from September through October 2020 from England found no differences in COVID-19 positivity rates between school teachers and other professions involving in-person interactions. Antibody testing data from the Schools Infection Survey conducted in March 2021 in the U.K. showed that the rate of antibody positivity rates in school staff were not higher than adult antibody positivity rates in the community.
Many of the studies described above took place in settings where multiple mitigation measures were in place to prevent COVID-19 spread in schools. Strategies varied widely among schools, but generally included combinations of classroom-based measures such as symptom screening, periodic testing, mask-wearing, physical distancing, cohorting of students, hand hygiene and improved ventilation in classrooms, as well as measures applied during peri-school activities such as transportation and extra-curricular activities.
Can we mitigate transmission in schools?
Studies from the United States and Europe show that when layered mitigation measures such as mask-wearing, social distancing, contact tracing and increased ventilation are in place, transmission in the context of in-person learning is significantly reduced. An analysis of contact tracing data from schools in North Carolina suggested that when mitigation measures were in place, transmission was limited. In August 2020, 56 of 115 North Carolina school districts joined a program to implement a suite of public health measures to prevent SARS-CoV-2 transmission. Those measures included universal mask-wearing for all individuals over 5 years of age, six-foot physical distancing, increased hand-washing, and daily symptom monitoring and temperature checks. In 11 participating school districts, during the first nine weeks of school reopening, when more than 90,000 students and staff attended school, there were 773 SARS-CoV-2 infections acquired in the community and 32 infections acquired within schools. Of 15 school-associated COVID-19 clusters, 11 were in schools that had not implemented the suite of measures. No instances of child-to-adult transmission of SARS-CoV-2 were reported within schools.
When layered mitigation measures are in place, transmission can remain low in schools even when there is substantial community transmission. In two Norwegian counties where schools reopened with multiple infection control measures in place, there was minimal transmission in primary schools despite moderate levels of community transmission from August to November, 2020. When cases that were linked to schools were investigated, which included systematic testing of all contacts twice during quarantine, less than 1% of child contacts and less than 2% of adult contacts tested positive for SARS-CoV-2. Similarly, a study conducted in rural Wisconsin during August 31–November 29, 2020, found that among 4,876 students and 654 staff members who participated in in-person learning in 17 K–12 schools, COVID-19 case rates among students and staff members were lower (3,453 cases per 100,000) than those in the county overall (5,466 per 100,000). Among 191 total cases in students and staff members, only one in 20 student cases was linked to in-school transmission and no infections among staff members were found to have been acquired at school. Data collected by the European Centres for Disease Control have also contributed to the general consensus that schools do not act as amplifiers of community transmission of SARS-CoV-2 when layered mitigation strategies are in place.
There are also examples of explosive disease spread in schools when mitigation measures are not sufficient. For example, in Israel, in May 2020, a large COVID-19 outbreak occurred in a high school 10 days after reopening. Two students who were symptomatic with COVID-19 attended in-person learning; face masks were not used, and classrooms were crowded. Among 1,161 students and 151 staff members who were tested for SARS-CoV-2, 153 students (13% attack rate) and 25 staff (17% attack rate) tested positive.
Limited implementation of mitigation measures has also been associated with COVID-19 outbreaks in sleepaway camps in Wisconsin and Georgia. A total of 597 campers and staff (median camper age 12 years, median staff age 17) attended the camp in Georgia; 260 of the 344 participants who were tested for SARS-CoV-2 were positive, for an overall attack rate of 44%. During one week of the investigation, cabin occupancy averaged 15 persons per cabin; the median cabin attack rate was 50% among 28 cabins that had one or more cases. This outbreak occurred in the context of substantial community transmission, and when relatively large cohorts shared cabins, masks were not universally used, and regular camp activities involved cheering and singing. A recent outbreak involving more than 125 people has been linked to a Texas camp for grades 6-12 where masks were optional, and 85 teenagers and adults tested positive for COVID-19 after attending a camp in Illinois that did not require masks indoors.
When transmission is linked to schools, it may be unclear whether transmission occurred in the classroom or during extra-curricular activities. During some activities, it may be difficult or impossible to practice or enforce mitigation measures including physical distancing and mask use. Several outbreaks have been linked to school sports. As one example, investigation into COVID-19 spread at a wrestling tournament in Florida revealed a minimum attack rate of 30% (38 of 126 tournament participants who were tested). Among 446 contacts of the 38 secondary COVID-19 cases, attack rates were highest among household members (30%) and wrestling team members who did not attend the tournament (20%). An estimated 1,700 in-person school days were lost due to isolation and quarantine of patients and contacts during this outbreak.
Have schools been a major contributor to community transmission?
Evidence from around the world over the past year suggests that the relationship between in-person education and community transmission is complex, but that school reopenings do not contribute significantly to community transmission when rates of community transmission are low and schools have mitigation measures in place. Higher rates of community transmission may increase risk of transmission in schools, but it is less clear that high transmission rates within schools drive community transmission rates.
In England, after the first national lockdown, the government conducted enhanced surveillance in educational settings that reopened. Surveillance in June and July 2020 included a median daily attendance of nearly 1 million students. Results showed that higher community incidence was strongly associated with increased risk of school outbreaks: the risk of an outbreak in schools increased by 72% for every five cases per 100,000 population increase in community incidence. In schools in Australia, there were few cases when community transmission was low from March to May 2020. Infections associated with schools peaked when community transmission was highest during July 2020, suggesting that cases in schools were driven primarily by transmission in the broader community. An analysis of data on school instruction modality and COVID-19 incidence in the community in Michigan and Washington during Fall 2020 showed school districts’ choices of hybrid versus full in-person instruction were not associated with COVID-19 spread in communities when case rates were low. An analysis of the instructional mode of schools versus COVID-19 hospitalizations in the U.S. suggested that in-person learning was not associated with increased COVID-19 hospitalizations in counties where community transmission rates were low; in counties where rates of COVID-19 hospitalization were higher prior to reopening, results were inconclusive. Data from 17 European countries, in which the return to school around mid-August 2020 coincided with a general relaxation of other mitigation measures in many countries, suggested that transmission in schools did not drive increases in cases observed across Europe in October 2020.
How might new variants affect transmission in schools?
Much of the available data on transmission patterns among children, in schools and between schools and the community, are from time periods before more highly transmissible SARS-CoV-2 variants emerged. Data now show that several new variants are more transmissible than older viral lineages. In particular, the alpha variant is estimated to be 50% more transmissible than older lineages. When the alpha variant emerged in the UK, the proportion of COVID-19 cases attributable to it rapidly increased in all regions of England, at comparable rates across age strata. Although cases caused by alpha included a larger share of under 20-year-olds than non-alpha cases for three weeks in November 2020, an analysis of transmission patterns over time suggested that the shifted age distribution was unlikely due to increased transmissibility among young people. Rather, that three-week period coincided with the second lockdown in England, during which schools remained open but other sectors were closed, so children may have had more contact and a higher risk of exposure to all variants compared to adults. Emergence of the alpha variant coincided with an increase in school-associated outbreaks in other places as well. For example, at a primary school in the Netherlands, one month after the first case caused by the alpha variant was identified at the school, tests of 818 teachers, students and families revealed that 123 people—nearly 15%—were infected. Of note, In January 2021 in the Netherlands, children attended school full-time, with full classes, and masks were not recommended in primary schools.
It is estimated that the delta variant is as much as 60% more transmissible than the alpha variant. There is not yet robust data on transmissibility by age group. Similar to the increased transmissibility of alpha among older people, reports of increased incidence among young people have emerged. For example, a serosurvey of the general UK population conducted in May and June 2021, when the delta variant was identified in over 90% of sequenced isolates, showed that the prevalence in those age 5-49 was 2.5 times higher than the prevalence in those age 50 years and above. Most infections in the younger group occurred in the unvaccinated population or those without a stated vaccine history. In June 2021, when Israel reported 125 new COVID-19 cases in a day – a number much higher than recent daily totals — the health ministry reported that 70% of the 125 infections were caused by the delta variant and that half of the infections occurred among children. In both Israel and the UK, vaccination rates among older people are high, while children under 12 remain unvaccinated. In addition, in both Israel and the UK, during the first six months of 2021, schools reopened for in-person learning while many other sectors remained closed. This likely changed contact patterns, increasing the chance of outbreaks in schools relative to other settings such as workplaces. The U.K. government has reported an increase in the number of school outbreaks after the emergence of the delta variant; data from January through June 2021, show that most outbreaks are associated with educational settings.
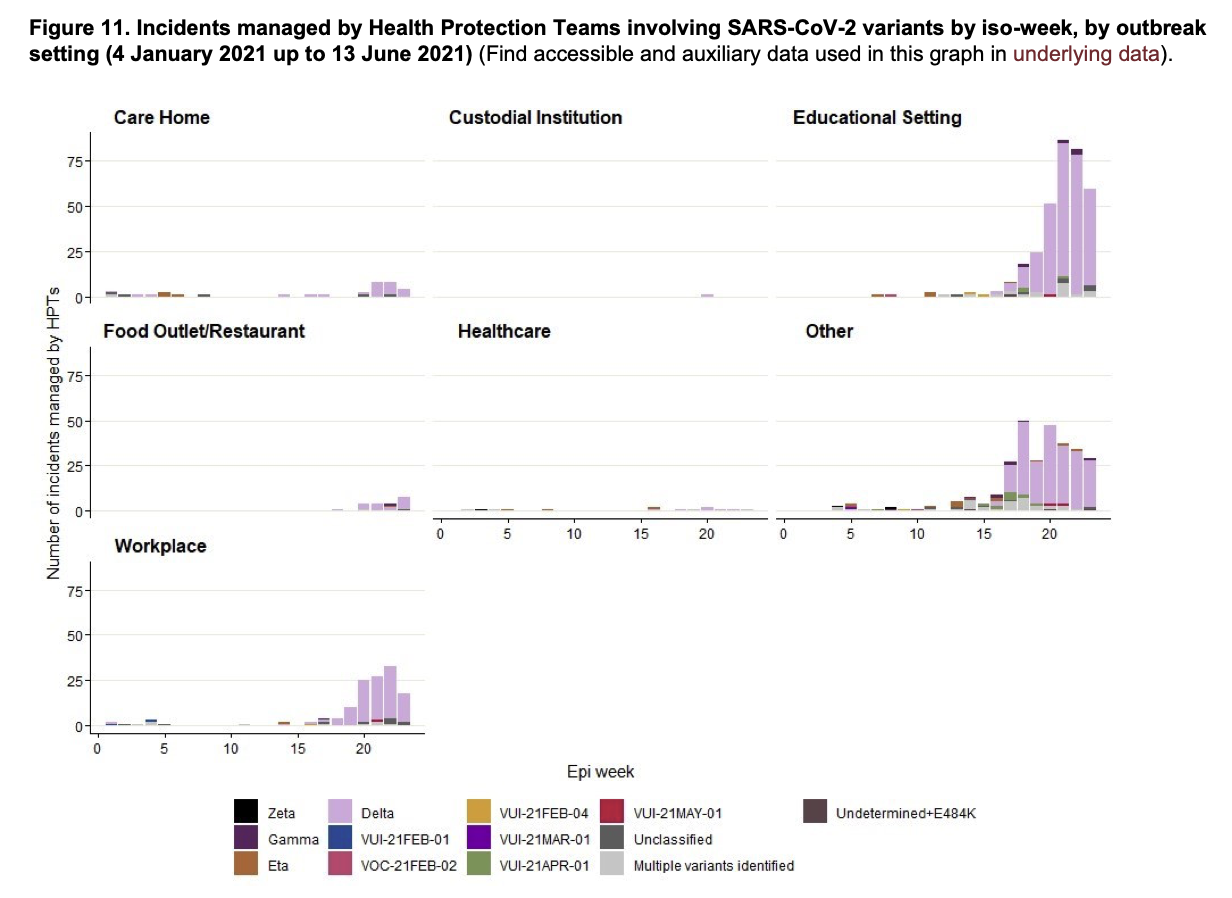
Source: Public Health England
Preliminary evidence suggests that widespread vaccination can also help to reduce COVID-19 case rates among people who remain unvaccinated. For example, in the Brazilian town of Serrana, 96% of adults were vaccinated as part of a study to measure the real-world effectiveness of the CoronaVac vaccine. Researchers documented an 80% decrease in symptomatic cases and 95% drop in deaths in the town in the two months after mass vaccination despite increases during the same period in 15 nearby cities. Although only 62% of Serrana’s 45,000 residents are adults, a similar drop in symptomatic infections occurred in unvaccinated children. Similarly, in the U.S. and Israel, COVID-19 case rates among children decreased as vaccination rates increased among adults, in some instances despite reopening of schools. An observational study of 177 communities in Israel estimated that, on average, for each additional 20% of individuals who are vaccinated, the test positivity among the unvaccinated population decreased approximately twofold in the following weeks. A limitation of the study is that policy measures, including a lockdown implemented during the study period, may have also contributed to the lower infection prevalence among unvaccinated persons, and the study included only community members who were part of a large health care network rather than all community residents. Although these early findings are promising, longer-term data are needed to understand the extent and duration to which widespread vaccination can limit the spread of COVID-19 among unvaccinated children.
Conclusion
Educating children safely has been a challenge throughout the pandemic. As vaccination coverage of adult populations in high-income countries reaches higher levels while children remain unvaccinated amid the emergence of increasingly transmissible variants of SARS-CoV-2, there is a relative increase in COVID-19 cases among children and in school settings. We must balance this against what the data show:
- Closing schools for in-person learning is deeply detrimental to the education and physical and mental health of children, as well as to the health and function of society.
- Abundant evidence shows that transmission and risk of outbreaks in schools can be reduced using layered mitigation measures. Per recent CDC guidance for COVID-19 prevention in K-12 schools, key measures include:
- Promoting vaccination
- Consistent and correct mask use
- Ventilation
- Physical distancing
- Screening testing and contact tracing to promptly identify cases, clusters, and outbreaks,
- Handwashing and respiratory etiquette, and
- Staying home when sick and getting tested
- Cleaning and disinfection
- Data suggest that although children can transmit SARS-CoV-2, this is less common than transmission among adults or from adults to children.
- Although children rarely get severely ill from COVID-19, some do, and we are still learning about impacts of new SARS-CoV-2 variants on disease severity and long-term sequelae in both children and adults.
- High rates of SARS-CoV-2 transmission in communities has been linked to risk of transmission and outbreaks in schools. In order to keep schools safe, community transmission should be monitored and kept to a minimum.
With the emergence of more transmissible variants, and with young children not yet vaccinated, layered mitigation measures in schools are critically important to keep schools open safely. The lower the rate of community spread, the lower the risk of cases in schools. Vaccination is our most potent tool to reduce community spread, including in the face of new variants — it is critical that everyone who can be vaccinated does so, and that global access to vaccinations is increased quickly and equitably.
Download a factsheet summarizing our key findings from the latest in-depth science review.
Myocarditis/Pericarditis FAQ
What are myocarditis and pericarditis and how frequently do they occur?
The estimated background rate of myocarditis among the U.S. general population is 1–10 cases per 100,000 persons annually. This is the frequency with which myocarditis is expected to occur in the absence of a new vaccination program. Viral infections are an important cause of myocarditis. Viral infections are an important cause of myocarditis. For example, myocarditis may occur in people with the flu and has been reported in association with COVID-19. More studies are needed to determine the frequency with which myocarditis occurs in people with COVID-19.
How frequent is myocarditis and pericarditis among those receiving the COVID-19 vaccines?
In the U.S., the Centers for Disease Control and Prevention (CDC) monitors the safety of vaccines, including COVID-19 vaccines, through several systems including the Vaccine Adverse Event Reporting System (VAERS). Since April 2021, there have been more than a thousand reports of cases of inflammation of the heart—called myocarditis and pericarditis— after mRNA COVID-19 vaccination (i.e., Pfizer-BioNTech, Moderna) in the United States. These reports are rare, given that hundreds of millions of vaccine doses have been administered.
As of June 28, 2021, the CDC and FDA confirmed 518 reports of myocarditis or pericarditis after over 324 million doses of COVID-19 vaccines were administered in the U.S. (December 14, 2020, through June 28, 2021), or about 1.6 cases per million COVID-19 vaccine recipients.
A review of people with myocarditis in Israel from December 2020 to May 2021 found that 148 people experienced myocarditis around the time of vaccination. Out of 5,401,150 people vaccinated with a first dose, 27 people had myocarditis around the time of the first dose; 11 were individuals with pre-existing conditions. Among 5,049,424 people vaccinated with a second dose, 121 people had myocarditis around the time of the second dose; of whom 60 had pre-existing conditions. This is approximately equivalent to 5 cases per million first-dose recipients and 24 cases per million second-dose recipients. Most people with myocarditis around the time of vaccination were hospitalized for up to four days, and 95% of cases were considered to be mild.
The review concluded that the risks for complications from COVID-19 outweighed the risks posed by side effects from the vaccine. The team from Israel also wrote, “There is some probability for a possible link between the second vaccine dose and the onset of myocarditis among young men aged 16 to 30. This link was found to be stronger among the younger age group, 16 to 19, compared to other age groups. This link became weaker the older the vaccinated individual is. In most cases, myocarditis took the form of a mild illness that passed within a few days.”
From the CDC and National Institute of Health, myocarditis and pericarditis after mRNA COVID-19 vaccination generally occur:
- Most often in male adolescents and young adults age 16 years or older
- More often after getting the second dose than after the first dose of one of these two mRNA COVID-19 vaccines (i.e., Pfizer, Moderna)
- Typically within several days after COVID-19 vaccination
- Among persons with certain medical conditions (e.g., infections, autoimmune disease) or recent medical procedures
When myocarditis does occur after mRNA vaccination, cases are generally mild. A review of cases in the U.S. through June 11 showed that among 304 hospitalized patients with known outcomes, clinical courses were generally mild, 95% had been discharged at time of review and none had died. For many patients, conservative treatment such as nonsteroidal antiinflammatory drugs was enough to resolve symptoms.
Should people still get the COVID-19 vaccine?
From the June 23, 2021, meeting of the Advisory Committee on Immunization Practices (ACIP), physicians and leading scientists presented the following reasons for people to still get the COVID-19 vaccine:
- Even with the decreasing number of people getting COVID-19 in the U.S. overall, recent projections of the number of people getting COVID-19 may increase substantially in the setting of low vaccination rates in some communities.
- Variants of concern (VOC), including the more transmissible B.1.617.2 (delta) variant, comprise an increasing proportion of SARS-CoV-2 lineages circulating in the U.S.
- From April to June 2021, adolescents and young adults aged 12–29 years had the highest COVID-19 incidence rates. In May 2021, 33% of cases occurred in persons aged 12–29 years.
- Although new cases per day have overall decreased in the U.S., COVID-19-associated hospitalization rates have remained stable in adolescents and young adults, and COVID-19-associated deaths continue to occur in adolescents and young adults.
- Post-COVID conditions, Multisystem Inflammatory Syndrome in Children (MIS-C), and Multisystem Inflammatory Syndrome in Adults (MIS-A) may occur among people with COVID-19 or who have recovered from COVID-19. The risk of MIS-C is much greater (estimated to be 316 per million infections among people under 21 years of age) than the risk of myocarditis around the time of vaccination (in the U.S., the highest reported rate of myocarditis within seven days of mRNA vaccination occurred among males aged 12−17 years after their second vaccine doses: 62.8 cases per million doses).
Take home message: Yes, people should still get vaccinated against COVID-19. COVID-19 vaccines are safe and effective. Millions of people in the United States have received COVID-19 vaccines under the most intense safety monitoring in U.S. history. The CDC recommends everyone ages 12 and older get vaccinated as soon as possible to help protect against COVID-19 and the related, potentially severe complications that can occur. Consult your health care provider if you have a history of myocarditis or pericarditis or have specific underlying risks for developing myocarditis or pericarditis before taking the COVID-19 vaccine.
Research Highlights
Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine
(NEJM, June 2021)
- The trial was conducted at 33 sites in the UK including more than 14,000 people, ages 18 to 84 years who were randomized 1:1 to vaccine or placebo. Among participants, 28% were aged 65 or older, 45% had coexisting illnesses and 95% were white. A larger trial is ongoing and will include more people of different races and ethnicities.
- Seven or more days after the second dose, there were 10 symptomatic COVID-19 cases in the vaccine group and 96 cases in the placebo group (VE: 89.7%). There were five severe COVID-19 cases in the placebo group (including 1 hospitalized patient) and none in the vaccine group at least seven days after the second dose. There were two COVID-19-related deaths among study participants – one in a vaccinated participant who developed COVID-19 symptoms seven days after the first dose and one in the placebo group. Efficacy was estimated to be 83% starting 14 days after the first dose and the median follow-up time in the study was three months after dose two.
- Vaccine efficacy did not differ for older compared to younger participants or among participants with or without coexisting illnesses.
- The estimated efficacy was 86% against the alpha variant compared to 96% against non-alpha variants; however, the trial was not designed to assess vaccine efficacy against specific variants and confidence intervals for these estimates were wide. Efficacy of the Novavax vaccine against the beta variant was estimated to be 51% in a separate trial.
- Vaccine side effects were mild to moderate and were more common in younger people and after the second dose, similar to the pattern observed for mRNA-based COVID-19 vaccines. Injection-site pain, fatigue, headache and muscle pain were the most commonly reported side effects. Fewer than 5% of vaccinated participants had a fever.
- The incidence of serious adverse events was similar in the vaccine and placebo groups (0.5% in each). One serious adverse event (myocarditis) was reported in a vaccine recipient, which occurred 3 days after the second dose and was considered to be a potentially immune-mediated condition; the patient resolved after 2 days in the hospital.
Prevention and Attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines
(NEJM, June 2021)
- This study, conducted between December 14, 2020, and April 10, 2021, enrolled health care, emergency response personnel and other essential workers in six U.S. states: Arizona, Florida, Minnesota, Oregon, Texas and Utah. Those who had evidence of SARS-CoV-2 infection before the study were excluded. Participants reported their demographic characteristics and vaccination status, were asked each month about potential exposures to SARS-COV-2 and were asked each week about any symptoms associated with COVID-19. If symptoms were present, the participant reported a number of clinical details about their illness. Each week, regardless of symptoms, participants provided a nasal swab to be tested by RT-PCR for SARS-CoV-2.
- The analysis included 3,975 participants. Most participants were female, 18 to 49 years of age, White and had no chronic medical conditions. 80% had received at least one dose of an authorized mRNA vaccine and 84% had received two doses by April 10. Two-thirds had received Pfizer and one-third Moderna.
- Among infected participants, three unvaccinated participants were hospitalized and no deaths were reported. The frequency of infection did not differ according to reported hours of potential virus exposure or PPE use.
- Estimated adjusted vaccine effectiveness against SARS-CoV-2 infection was 91% (95% confidence interval [CI], 76 to 97) with full vaccination and 81% (95% CI, 64 to 90) with partial vaccination.
- The mean viral RNA load was 40% lower (95% CI, 16.3 to 57.3) with at least partial vaccination than with no vaccination. The risk of viral RNA detection for more than 1 week was 66% lower with at least partial vaccination.
- Among participants with SARS-CoV-2 infection, 25% of those who were partially or fully vaccinated reported febrile symptoms, compared with 63% of those who were unvaccinated. Vaccinated participants also reported 6.4 fewer total days of symptoms (95% CI, 0.4 to 12.3) and 2.3 fewer days spent sick in bed with Covid-19 (95% CI, 0.8 to 3.7) than unvaccinated participants.
- Limitations include that the follow-up period was brief, participants were not demographically diverse, and the differential effects of partial versus full vaccination on risk of breakthrough infection could not be determined because the number of breakthrough infections was small.
Suggested citation: Cash-Goldwasser S, Jones SA, Wu AC, Subramaniam HL and Frieden TR. In-Depth COVID-19 Science Review July 16, 2021. Resolve to Save Lives. 2021 July 16. Available from https://preventepidemics.org/covid19/science/review/





