What do we know about the Novavax vaccine?
Vaccine types
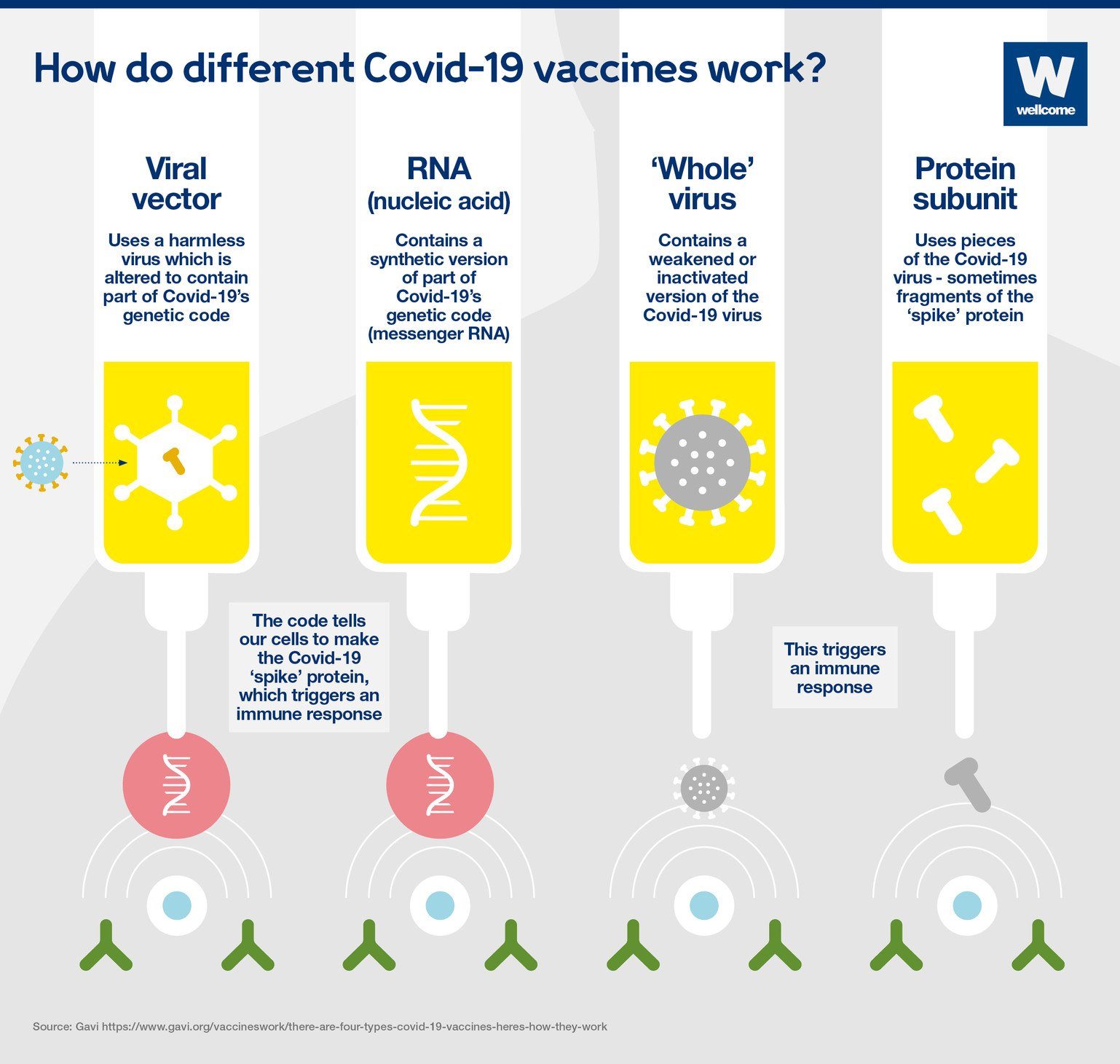
Vaccines prime the immune system to recognize pathogens (viruses, bacteria, or other microorganisms that cause disease) and thus prevent infection or reduce the severity of disease if exposed to those pathogens. During an infection, the immune system reacts to antigens, or parts of the pathogen that stimulate the immune system. The immune system is also able to recognize antigens that are separate from a whole pathogen. Our three newer types of vaccines – viral vector, nucleic acid and subunit vaccines – take advantage of this fact.
Source: Welcome.org
Whole-pathogen vaccines
Traditional vaccines contain whole pathogens. In some vaccines, the pathogen is inactivated (killed); in others, it is live-attenuated (alive but weakened). Live-attenuated vaccines, such as the measles, mumps and rubella vaccine, tend to induce strong and long-lasting immunity. However, because there is a tiny risk these vaccines can cause infection, they are not safe for some immunocompromised people. Inactivated vaccines, including vaccines against rabies and influenza, are incapable of causing disease, but may not stimulate the immune system as strongly and can require boosters. All three whole-virus COVID-19 vaccines listed for emergency use by WHO — Sinopharm, Sinovac and Covaxin — contain inactivated SARS-CoV-2. They also contain adjuvants, or substances that enhance immune responses to antigens.
To produce whole-pathogen vaccines, large quantities of the pathogen must be grown, harvested and processed. Although manufacturing these vaccines can be complicated and challenging to scale up quickly, many vaccine manufacturers have experience with this platform and possess the necessary technology. Sinopharm, Sinovac and Covaxin can be kept at refrigeration temperatures and do not require ultra-cold storage.
Nucleic acid vaccines
Nucleic acid vaccines introduce fragments of a pathogen’s genetic material to human cells. The cells use that genetic material to produce pathogen proteins which function as antigens. The two nucleic acid COVID-19 vaccines listed by WHO, Moderna and Pfizer, both use SARS-CoV-2 genetic material in the form of mRNA. Although mRNA vaccines are a relatively new technology and no mRNA vaccine had been authorized before the COVID-19 pandemic, several mRNA vaccine candidates had been developed and tested in human trials over decades before the pandemic.
Nucleic acid vaccines do not contain infectious material and are believed to be safe for immunocompromised people. The Moderna and Pfizer vaccines are highly immunostimulatory and do not contain adjuvants. The production of mRNA vaccines is both novel and expensive. However, with sufficient resources, the process can be scaled up rapidly with relative ease. Another advantage of mRNA vaccines is that they can quickly be reformulated to target new SARS-CoV-2 variants. A downside is that both the Moderna and Pfizer vaccines require very cold temperatures for long-term storage.
Viral vector vaccines
Viral vector vaccines use harmless viruses to carry pathogen genetic material into human cells. Similar to nucleic acid vaccines, the cells use that genetic material to produce proteins which function as antigens. There are now three vectored COVID-19 vaccines listed by WHO: AstraZeneca, J&J and Covishield (the AstraZeneca vaccine made by the Serum Institute of India). Prior to the COVID-19 pandemic, many vectored vaccines had been developed and tested in human clinical trials, and vectored Ebola vaccines had been authorized for use.
Similar to mRNA vaccines, vectored vaccines are believed to be safe for immunocompromised people. Vaccine manufacturers have more experience with the technology required to produce vectored vaccines than that required to produce mRNA vaccines, and the process is cheaper. However, manufacturing vectored vaccines is relatively complex, time-consuming and subject to setbacks. The three vectored COVID-19 vaccines listed by WHO can be kept at refrigerator temperature.
Subunit vaccines
Subunit vaccines contain only specific pathogen components selected for their antigenic potential. Many of our vaccines — against hepatitis B, human papillomavirus (HPV), influenza, pertussis, shingles, and bacteria that cause pneumonia and meningitis — are subunit vaccines. These vaccines cannot cause disease and are safe for immunocompromised people and may cause fewer side effects. For example, the pertussis vaccines introduced in the 1940s contained whole inactivated bacteria and frequently caused reactions. This contributed to low vaccination rates as people avoided vaccination. The pertussis vaccine licensed in the U.S. today is a subunit vaccine that causes fewer reactions.
There are several types of subunit vaccines. In the Novavax vaccine and other subunit COVID-19 vaccines in development, the subunit is a protein. The production of protein subunit vaccines is somewhat similar to the production of whole-pathogen vaccines: the antigens must be grown, harvested and processed. Although this process is relatively complex, difficult to scale up and susceptible to setbacks, many manufacturers have extensive experience producing subunit vaccines. In fact, WHO listed two versions of the Novavax vaccine for emergency use, one of which is the Covovax vaccine, which is the same formulation as Novavax but manufactured by the Serum Institute of India. In addition, subunit vaccines can be cheaper and easier to produce than other vaccine types. Another advantage of subunit vaccines is that they are relatively stable and can be stored at refrigeration temperatures for long periods of time. This is a major advantage to vaccine delivery and administration efforts in low- and middle-income countries. One downside of the antigenic precision of subunit vaccines is that they may not stimulate the immune system strongly and may require booster doses and/or the use of adjuvants.
Subunit vaccines against COVID-19
There are currently more than 130 COVID-19 vaccines in clinical development, including more than 40 subunit vaccines. Although only two subunit vaccines (one formulation) are listed for emergency use by WHO, 10 COVID-19 subunit vaccines have been authorized for use in at least one country. The Novavax vaccine was authorized for use in Indonesia and the Philippines prior to WHO listing. Examples of other COVID-19 subunit vaccines authorized in at least one country include the Abdala vaccine (made in Cuba and authorized in Cuba, Nicaragua, Venezuela and Vietnam), the EpiVacCorona vaccine (made in Russia and authorized in Russia and Turkmenistan) and the Zifivax vaccine (made in China and authorized in China, Uzbekistan and Indonesia). These authorizations have filled country or regional needs for increased vaccine access. For many of these vaccines, clinical trial data on vaccine performance are not publicly available. The Novavax vaccine is the subunit COVID-19 vaccine about which we know the most.
Novavax: production, adjuvant, safety and efficacy
Production
When designing the Novavax vaccine, scientists selected a SARS-CoV-2 gene to target: the S-gene, from which the spike protein is produced. To produce the Novavax vaccine, that gene is inserted into a virus that infects insects, formally known as a baculovirus, which is then used to infect moth cells. Each infected moth cell uses the S-gene to create many spike proteins. Those spike proteins are extracted, purified and combined with an adjuvant called Matrix-M.
Adjuvants including Matrix-M
Adjuvants are necessary to achieve sufficient immune responses to many vaccines. They can improve the efficacy of vaccines in people with weaker immune responses, including older adults. For example, one flu vaccine approved and recommended in the U.S. for people over age 65 is an adjuvanted vaccine. Adjuvants can also help scale up vaccination during a pandemic by reducing the amount of antigen needed per dose to induce sufficient immunity. During the 2009-10 H1N1 influenza pandemic, it took months to produce enough vaccine doses to cover even half of the U.S. population. A 2010 report to the U.S. president recommended additional research on adjuvants so that in future pandemics, the vaccine supply could be expanded by using less influenza virus material per dose.
Since the first adjuvanted vaccines were developed nearly a century ago, we have learned a great deal about the efficacy and safety of adjuvants. Aluminum salt (alum) adjuvants have been used in vaccines since the 1930s and continue to be the most commonly used adjuvants today. In recent decades, the development of new adjuvants and the reformulation of antigen-adjuvant combinations has increased adjuvant availability, made vaccines more effective, and reduced vaccine adverse events. The U.S. Food and Drug Administration (FDA) does not license vaccine adjuvants in isolation because the function of each adjuvant depends on the antigen with which it is combined. Rather, the safety and efficacy of every adjuvanted vaccine must be demonstrated in clinical trials. Adjuvanted vaccines can cause higher rates of reactions than non-adjuvanted vaccines, but these reactions are mostly mild and transient. The size of pre-licensure clinical trials may not be sufficient to detect rare adverse events, so it is essential to monitor vaccine safety after a vaccine is made available to the general population. The U.S. Centers for Disease Control and Prevention uses several systems to monitor vaccine safety. It is these and similar systems in other countries which detected rare severe adverse events associated with mRNA and vectored COVID-19 vaccines (none of which contain adjuvants).
The safety of vaccine adjuvants was scrutinized during the 2009 H1N1 influenza pandemic when millions of doses of adjuvanted H1N1 vaccines were administered around the world. One of the H1N1 vaccines given in Europe, Pandemrix, contained the newer adjuvant AS03. After vaccine rollout, countries in northern Europe reported an increase in reports of narcolepsy, a chronic neurologic disorder characterized by excessive daytime sleepiness. Investigations into the association between H1N1 vaccination and narcolepsy was complicated by several factors. First, the cause of narcolepsy is not known. Second, in China, H1N1 infection was associated with an increased risk of narcolepsy. Third, the H1N1 vaccine used in Canada and Brazil also contained AS03, but reports of narcolepsy in those countries did not increase. In 2018, a large analysis of safety data on adjuvanted H1N1 vaccines from multiple countries did not detect an association between vaccination and narcolepsy. It is clear that vaccine safety surveillance systems effectively detected a very rare adverse event (narcolepsy occurred in less than one out of 10,000 Pandemrix recipients) after vaccine rollout. It is not clear what caused increased reports of narcolepsy in northern Europe during the H1N1 pandemic and vaccination campaign.
Matrix-M is a proprietary adjuvant produced by the Novavax company which is made from an extract of the Chilean soapbark tree (Quillaja saponaria). It has never been included in any licensed vaccine. However, pre-clinical and early-phase human trials of influenza vaccines prior to the COVID-19 pandemic demonstrated that Matrix-M has an acceptable safety profile, augments immune responses and allows the use of less antigen per vaccine dose.
The adjuvant AS01 also contains extract from the Chilean soapbark tree (in AS01, this extract is formulated into a compound called QS-21). AS01 is the adjuvant in the Shingrix vaccine, a subunit vaccine that prevents shingles, and in the RTS,S/AS01 vaccine, a subunit vaccine against malaria. In October 2021, the RTS,S/AS01 vaccine became the first malaria vaccine in history recommended for widespread use. This groundbreaking achievement illustrates the critical role an adjuvant can play. Over decades of vaccine development, the antigenic component of the vaccine (RTS,S) was formulated with several different adjuvants including, eventually, AS01. The version with AS01 has an acceptable safety profile and provides more protection against malaria than other RTS,S-adjuvant combinations. A new malaria vaccine, adjuvanted with Matrix-M instead of AS01, has now been developed. This is partially due to concerns about vaccine supply if there is total reliance on AS01, which is proprietary and produced by one manufacturer. A clinical trial in Burkina Faso showed that the vaccine with Matrix-M was safe and highly effective.
Although clinical trials on Novavax and other vaccines containing Matrix-M have demonstrated efficacy without safety concerns, it will be important – as for every vaccine – to monitor the safety of Novavax with robust post-marketing surveillance systems.
Safety and efficacy
Several clinical trials have evaluated the safety and efficacy of the Novavax vaccine as a two-dose primary series given 28 days apart. In a trial with 30,000 adults in Mexico and the U.S. during early 2021, the efficacy of Novavax to prevent mild COVID-19 was 90%, and the efficacy to prevent moderate or severe disease was 100%. Most sequenced isolates were the Alpha variant of SARS-CoV-2. Adverse events occurred more frequently among vaccine than placebo recipients, but most events were mild to moderate and transient. Severe adverse events were very rare and occurred equally in the vaccine and placebo groups. Because other COVID-19 vaccines were authorized by the time this trial began, few older adults enrolled and it was not possible to determine vaccine efficacy in that high-risk population. A trial conducted in late 2020 with more than 20,000 adults in the United Kingdom provided more data on older adults. Overall, vaccine efficacy to prevent symptomatic COVID-19 was 90%. Among those who were 65 years of age and older, efficacy was 89%. Efficacy was 86% against the Alpha variant and 96% against non-Alpha strains. Although more people in the Novavax than placebo arm reported mild vaccine side effects, there were no safety concerns in this trial.
In a Novavax trial that enrolled 5,000 adults in South Africa, 90% of sequenced isolates were the Beta variant. Among participants without previous COVID-19, vaccine efficacy to prevent mild or moderate COVID-19 was 43%. Among those without HIV, efficacy was 51%. These numbers suggest significantly less protection than observed in other trials. Of note, trials of other vaccines in South Africa when the Beta variant predominated showed slightly (J&J) or significantly (AstraZeneca) reduced efficacy compared with results from sites where the Beta variant was uncommon. That said, there is evidence that vaccine protection against severe disease is better-maintained across variants than protection from mild disease: estimates of J&J efficacy against severe disease from sites in South Africa, South America and the U.S. were similar. The AstraZeneca and Novavax trials in South Africa did not yield estimates of efficacy against severe disease because there were too few cases of severe COVID-19 in those trials.
There are no clinical data on the protection offered by the Novavax vaccine against the Delta or Omicron variants. When Delta emerged, one study analyzed antibody responses to a Novavax booster given six months after a Novavax primary series. According to a press release from the company, booster doses were well-tolerated. Antibody responses after boosting were more than four times higher than antibody responses after the primary series, and all boosted participants developed robust antibody responses against the Alpha, Beta and Delta variants. It is not possible to estimate real-world Novavax effectiveness against the Delta variant from these data. Recently, the company announced that it is testing its vaccine against the Omicron variant in the lab and has started developing an Omicron-targeted vaccine.
The question of whether COVID-19 vaccines can be mixed and matched is important as the number of authorized COVID-19 vaccines increases, the vaccines available in many places change over time, and there is increasing imperative to boost as time passes and new variants emerge. The Cov-Boost trial studied the safety and immunogenicity of seven different boosters, including Novavax, among participants 30 years of age and older who had received an AstraZeneca or Pfizer primary series. There were no safety concerns. All booster vaccines (with the exception of the whole-virus inactivated Valneva vaccine) effectively stimulated immune responses. Among those with an AstraZeneca primary series, Novavax boosters increased immune responses at least as much as AstraZeneca boosters. Among those with a Pfizer primary series, Novavax boosters increased immune responses but not as much as Pfizer boosters. These results support mixing and matching vaccines, which provides options for adapting regimens to local supply. However, the real-world effectiveness of Novavax boosters combined with any primary series is unknown.
In summary, available data suggest that the Novavax vaccine is highly protective against COVID-19 caused by the Alpha variant and less protective against the Beta variant. The Beta variant is known for immune evasion, and other vaccines have demonstrated reduced efficacy against it. Data on other vaccines suggest that protection against severe disease may be maintained across variants; however, we lack data on Novavax against newer variants including Delta and Omicron. Immunologic data support boosting with Novavax even if a different vaccine was given as the primary series, though the real-world protection offered by a Novavax booster may not be as strong as the protection offered by an mRNA booster.
Novavax regulation and supply
Despite early enthusiasm about and major global investments in Novavax’s COVID-19 vaccine in 2020, multiple manufacturing and regulatory setbacks caused the vaccine to fall far behind other vaccine candidates as the pandemic progressed.
Novavax had set a production target of over 2 billion doses by the end of 2021. By comparison, Moderna’s target was 1 billion and Pfizer’s target was 3 billion. At least 700 million Novavax doses have been purchased directly by countries and a significant portion of the COVAX vaccine inventory was supposed to be Novavax – in early 2021, the company had promised more than 1 billion doses to COVAX. Without emergency use listing, this promise could not be fulfilled, so the recent decision from WHO is a major step forward. Another step forward was the December 21 recommendation of the European Medicines Agency to authorize Novavax for use in the European Union. The number of doses available and the rapidity with which they are delivered and administered remains to be seen.
Novavax is not the only subunit vaccine expected to boost global vaccine supply. Ten subunit COVID-19 vaccines have been authorized in at least one country, and many others are in development. Production projections are publicly available for some of these vaccines. For example, the company that makes the Clover subunit vaccine – which has not received emergency use listing from WHO – has announced plans to produce 1 billion doses annually and has signed a 400 million dose agreement with COVAX.
Conclusion
The Novavax COVID-19 vaccine was granted emergency use listing by WHO a mere three weeks after the emergence of a new, highly transmissible variant that has caused global COVID-19 cases to skyrocket. Omicron has underscored the need for increased global vaccine access more than ever. The Novavax vaccine and other subunit COVID-19 vaccines may be an important part of the solution. As we have previously written, ensuring a sufficient and reliable supply of COVID-19 vaccines globally has been a major, ongoing problem. Major vaccine manufacturers have fallen far short of their stated production targets and efforts to increase mRNA vaccine production capacity have been too little and come too late. Many vaccine manufacturers have experience producing subunit vaccines like Novavax, which offers the opportunity to increase production capacity. Logistical bottlenecks to getting shots into arms in low- and middle-income countries include the need for cold chain storage; subunit vaccines may be stored at refrigerator temperature.
Rolling out the Novavax vaccine and carefully monitoring its safety will determine whether the vaccine can cause very rare severe adverse effects, as has been found for mRNA (myocarditis) and vectored (a serious blood clotting disorder) COVID-19 vaccines. Clinical trials have shown that the Novavax vaccine is safe and effective against some SARS-CoV-2 variants and increases immunity effectively when used as a booster. Time and real-world data will teach us about the effectiveness of Novavax against Delta, Omicron and future variants. From what we know now, Novavax is a safe and effective vaccine that will improve global vaccine access.
Suggested citation: Cash-Goldwasser S and Frieden TR. In-Depth COVID-19 Science Review December 22, 2021. Resolve to Save Lives. 2021 December 22. Available from https://preventepidemics.org/covid19/science/review/





