Protecting the Immunocompromised: The Evidence for an Additional Dose of COVID-19 Vaccine
What is immunocompromise?
Immunocompromise, the state of having a compromised or weakened immune system, can be caused by an inherited condition (primary immunodeficiency) or acquired because of a disease or treatment (secondary immunodeficiency). The extent to which the immune system is compromised varies by condition. Some conditions — including several autoimmune diseases — may have relatively little impact on immune function, but the treatments given to prevent the immune system from attacking the self may cause significant immunosuppression.
As we have previously written, the immune response to a disease or vaccine involves an array of organs, cells and molecules. The adaptive immune response, which “remembers” previous exposures to a given pathogen, is composed of B- and T-cells. Some conditions and medications compromise specific components of the immune response, whereas others have effects on multiple parts of the immune system. For example, in HIV infection, T-cells are attacked, whereas those who take the medication rituximab — a therapy for some autoimmune diseases and cancers — have impaired B-cell responses. In contrast, people undergoing stem cell transplants receive treatments that reduce the entire adaptive immune response to prevent rejection of transplanted cells.
The number of immunocompromised adults in the U.S. is unknown. In the 2013 National Health Interview Survey, 2.8% of approximately 35,000 respondents over age 18 reported current immunosuppression. Applying that percentage to the U.S. adult population suggests that there might be approximately 6 million immunocompromised adults in the U.S. Given advances in immunosuppressive therapies, possible under-reporting in surveys, and increases in the number of people on such treatments, this may be an underestimate.
What do we know about non-COVID-19 vaccines for immunocompromised people?
Some vaccine recommendations for people who are immunocompromised differ from recommendations for the general population in the number, type or timing of doses. Additional doses may be included in the primary vaccine series (e.g., human papillomavirus [HPV] vaccine). Other vaccines (e.g. pneumococcal vaccines) are recommended routinely for the general population at certain ages and then re-administered at other ages to some with immunocompromise. For other vaccines, a higher dose (e.g., hepatitis B) or specific vaccine formulation (e.g meningitis), are recommended. There may also be vaccination timing recommendations. For example, certain vaccines should be administered prior to planned future immunosuppression (e.g., spleen removal or solid organ transplantation), and childhood vaccines should be re-administered after immune system recovery in some people (e.g., stem cell transplant recipients). Temporarily discontinuing immunosuppressive medication — if safe to do so — for a period of time after vaccination may result in more robust immunity. An example of supporting evidence is a study which showed that temporary discontinuation of the immunosuppressive drug methotrexate for two weeks after flu vaccination improved the immune response to vaccination among patients with rheumatoid arthritis.
There are unique vaccine safety concerns for immunocompromised populations. One is that live vaccines — including the vaccines for chickenpox, measles, mumps and rubella [MMR], as well as some flu vaccines — should generally be avoided in those with significant immunocompromise. Even though the viruses in such vaccines are attenuated, or weakened, they could theoretically cause infection. Another concern is that stimulating the immune system with vaccination may cause complications. For example, in someone with a solid organ transplant, immune stimulation could theoretically contribute to rejection of the transplanted organ. However, there is no evidence to support a causal relationship between vaccination and transplanted organ rejection. As always, the risk of vaccine adverse events must be balanced against the benefit of vaccination. Vaccines can prevent not only severe infections but also complications unique to immunocompromised people. For example, if a severe infection occurs in someone taking an immunosuppressive medication, it may be necessary to reduce that medication so the immune system can respond to the infection. However, this can increase the risk of transplanted organ rejection or a flare-up of an autoimmune condition.
Recommendations for vaccination of immunocompromised persons are provided by the Advisory Committee on Immunization Practices (ACIP) to the Centers for Disease Control and Prevention (CDC) and the Infectious Diseases Society of America (IDSA) in the U.S. Many of these recommendations are based on laboratory studies that have measured the immune response to infection or vaccination. Data on real-world vaccine effectiveness among immunocompromised persons are limited for several reasons. First, the spectrum of immunocompromise is vast, and study findings may not be generalizable across different conditions. Second, clinical trials often exclude immunocompromised persons. Third, post-marketing studies (conducted after a vaccine is in widespread use) may include only small numbers of immunocompromised persons, making it difficult to draw conclusions, especially about vaccine safety. Fourth, it can be difficult to disentangle the effects of vaccinating an immunocompromised population from the effects of vaccinating the general population. For example, after vaccination against pneumococcal bacteria was recommended for all children under two years of age, the rate of serious pneumococcal disease among children with sickle cell disease fell by 93%, which may have been due to vaccination in those with sickle cell disease as well as to spillover protection (“herd immunity”) from vaccinating other children.
What are the risks of COVID-19 among people who are immunocompromised?
Some immunocompromised people are at higher risk of severe disease and death from COVID-19 than people with normal immune systems. Early data from China showed that a disproportionately high number of people with severe COVID-19 had cancer. In an early cohort study from the UK — one of the largest studies on risk factors for death due to COVID-19, with 17 million participants and more than 10,000 COVID-19 cases — the risk of death was higher among those with:
- Autoimmune diseases (rheumatoid arthritis, lupus or psoriasis),
- Several cancers (especially if diagnosed within the past 5 years),
- Solid organ transplantation,
- A history of end-stage kidney disease or dialysis, and
- Several other immunocompromising conditions.
Since then, multiple studies have attempted to quantify risks associated with different immunocompromising conditions and medications. However, it is not clear exactly which types of immunocompromise might lead to specific degrees of COVID-19 risk. This is in part because of the broad spectrum of immunocompromise, even among people with the same condition. For example, medication-induced immunosuppression commonly varies over time, such as in patients who receive solid organ transplants, who are typically given high-intensity “induction” therapy around the time of transplant and then later transitioned to lower-intensity (less immunosuppressive) “maintenance” therapy to reduce the longer-term risks of infection. In addition, confounding factors make it difficult to tease apart the relationship between being immunocompromised and COVID-19 risk: many immunocompromising conditions are associated with older age or with other risk factors for severe COVID-19. In some studies, much of the risk of COVID-19 associated with being immunocompromised may be explained by other factors.
Evidence also suggests that COVID-19 patients who are immunocompromised pose specific risks for transmission to others. Some immunocompromised COVID-19 patients have been found to shed the virus for months. Also, persistent infection in immunocompromised patients can result in accelerated evolution of the virus and the accumulation of mutational changes similar to those found in known variants of concern. In addition, compared with people who have normal immune systems and get COVID-19, those who are immunocompromised and have COVID-19 are more likely to transmit infection to their household members.
How well do COVID-19 vaccines protect immunocompromised people?
As is common in new drug or vaccine trials, the COVID-19 vaccine trials conducted in the U.S. excluded immunocompromised persons. Immunocompromised people may be excluded because of potential risks to this vulnerable population associated with a new, untested pharmaceutical product, and in part because inclusion could complicate interpretation of results for the general population without producing robust estimates of vaccine effectiveness among immunocompromised persons. As a result, to learn how well COVID-19 vaccines protect immunocompromised people, we must look to laboratory studies of the immune responses to COVID-19 vaccines, observational studies of vaccine effectiveness in persons who are immunocompromised and information about breakthrough infections (COVID-19 infections among fully vaccinated persons).
1) Laboratory studies
Studies have found that among immunocompromised persons who have received two doses of mRNA vaccine (Pfizer or Moderna), fewer develop antibodies, and among those who do, the levels of antibodies developed are lower than among persons without immunocompromise, suggesting a lower level of protection after vaccination. The strength of antibody response varies by condition but is particularly diminished among more severely immunocompromised groups: patients with solid organ transplants or hematologic (blood) cancers and those on immunosuppressive medications. The figure below shows the percentage of people with antibody responses to COVID-19 vaccination among four categories of immunocompromised people compared to antibody responses among healthy people. Each dot in the figure represents an estimate from one study.
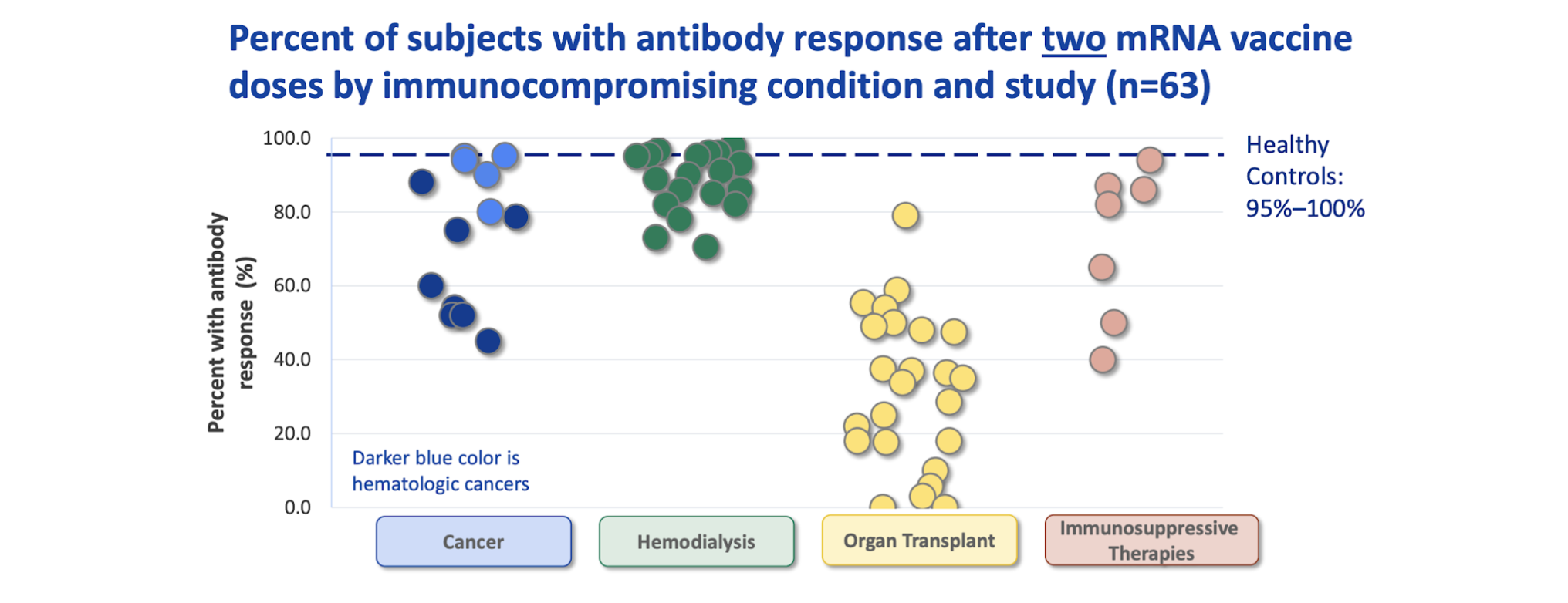
Source: ACIP
Notable findings from some studies on COVID-19 vaccines in immunocompromised people include:
- In a preprint prospective cohort study of 107 health care workers and 489 immunocompromised people who were fully vaccinated, 98% of fully vaccinated health care workers seroconverted — developed detectable antibodies — after vaccination. In contrast, the proportions of immunocompromised people with detectable antibodies were:
- 37% for solid organ transplant,
- 55% for hematological malignancies,
- 82% for solid organ tumor,
- 84% for autoimmune diseases, and
- 94% for HIV.
- Among 658 solid organ transplant recipients who received complete mRNA vaccine series, 46% had no detectable antibodies. Poor antibody responses were associated with the use of specific immunosuppressive medications known as antimetabolites.
- Among 244 dialysis patients who received two doses of the Pfizer vaccine, 91% had detectable antibodies. Those who did not were older or on immunosuppressive medication.
- Among 404 patients with rheumatic and musculoskeletal diseases who received a complete mRNA vaccine series, 94% tested positive for antibodies. Lower rates of antibody response were observed among patients taking specific medications: mycophenolate (73% seropositive) or rituximab (26% seropositive).
It is difficult to extrapolate the level of protection against infection or severe disease from antibody data. Studies are underway to learn about immune correlates of protection against COVID-19, including antibody levels that may predict protection from disease. Such predictions may be even less accurate in people who are immunocompromised. Also, some of the above studies have limitations that further limit conclusions, including lack of a comparison group of immunocompetent persons and no data on B- and T-cell responses. In addition, it can be problematic to compare antibody data across studies that use different laboratory assays with variable performance characteristics.
2) Observational studies
Observational studies have found reduced effectiveness of COVID-19 vaccines in some immunocompromised people. For example, a preprint case-control study that included 1,210 adults hospitalized with COVID-19 in the U.S. showed that mRNA vaccines were 59% effective against hospitalization among immunocompromised patients (versus 91% in immunocompetent patients). A retrospective cohort study in Israel that included data on 1.1 million vaccinated people found that Pfizer vaccine effectiveness against symptomatic COVID-19 was 75% among immunocompromised people versus 94% in immunocompetent persons.
3) Breakthrough infections
Studies have also shown that immunocompromised persons account for a large proportion of hospitalized breakthrough cases. In a study from Israel, of 152 patients hospitalized with COVID-19 at least seven days after receiving their second dose of Pfizer vaccine, 40% were immunocompromised. In a preprint study on mRNA vaccine effectiveness among 1,210 participants from the U.S., there were 45 breakthrough hospitalizations, of which 20 (44%) occurred in people with immunocompromise. Another study from the U.S. showed that the risk of breakthrough infection and associated death was much higher among solid organ transplant recipients than among the general population: among 18,215 fully vaccinated solid organ transplant recipients, there were 151 breakthrough infections and 14 associated deaths (9.3%). In comparison, of 101 million fully vaccinated adults in the U.S. through April 30, 2021, CDC reported 10,262 breakthrough infections and 160 associated deaths (0.00016%). These data suggest that not only are immunocompromised people at higher risk for breakthrough infections, but also that when such infections do occur they are more likely to cause severe disease.
A major limitation of these studies is that it is not possible to draw conclusions about how much protection or risk is associated with specific types of immunocompromise. Definitions of immunocompromise varied among studies and each included participants with a wide range of conditions. In addition, these studies focused on mRNA vaccines (data cannot be extrapolated to other vaccine types), and they were conducted before widespread circulation of the Delta variant (it is unclear how the Delta variant may change vaccine effectiveness among immunocompromised people). However, the sum of the evidence indicates that COVID-19 vaccines do not protect some immunocompromised persons as effectively as people with healthy immune systems.
What can we do to better protect immunocompromised persons from COVID-19?
There is evidence that a third dose of mRNA vaccine can lead to seroconversion in a significant proportion of immunocompromised people who did not seroconvert after their second dose, along with increased antibody levels.
- A study recruited 30 solid organ transplant recipients who had gotten two doses of an mRNA vaccine and were given a dose of Pfizer, Moderna or J&J by their providers an average of 67 days after their second vaccine dose. Of 24 people who were seronegative after a second dose, 8 (33%) seroconverted after a third dose. Six people with low antibody titers after a second dose had higher levels after a third dose.
- Among 101 solid organ transplant recipients who received two doses of Pfizer vaccine and then got a third dose an average of 61 days after their second dose, the proportion of people who had antibodies was 0% before the first dose, 4% before second dose, 40% before the third dose and 68% four weeks after the third dose. Among 59 patients seronegative before their third dose, 26 (44%) seroconverted after the third dose. Patients who were already seropositive after their second doses had large increases in antibody titers after the third dose. Patients who were still seronegative after three doses tended to be older and have higher degrees of immunosuppression.
- A preprint study looked at responses to third vaccine doses among patients on hemodialysis who received three doses of Pfizer vaccines. Among 56 patients who had a suboptimal immune response after two doses, nearly two thirds had robust immune responses after a third dose.
The extent to which the measured immune responses of immunocompromised persons may be expected to change over time in the absence of a third vaccine dose is unknown, and the above studies lacked control groups. One study addressed this issue by randomizing 120 transplant recipients who had received two doses of the Moderna vaccine to receive either a third dose of Moderna or a saline placebo injection two months after the second vaccine dose. Two months after the third injection, 33 of 60 patients (55%) in the Moderna group, compared with 10 of 57 patients (18%) in the placebo group, had antibody levels above a pre-specified threshold. T-cell counts were also higher in the group that received a third dose of vaccine. The figure below shows immune responses before and after a third vaccine dose in the Moderna (mRNA-1273) group and in the placebo group.
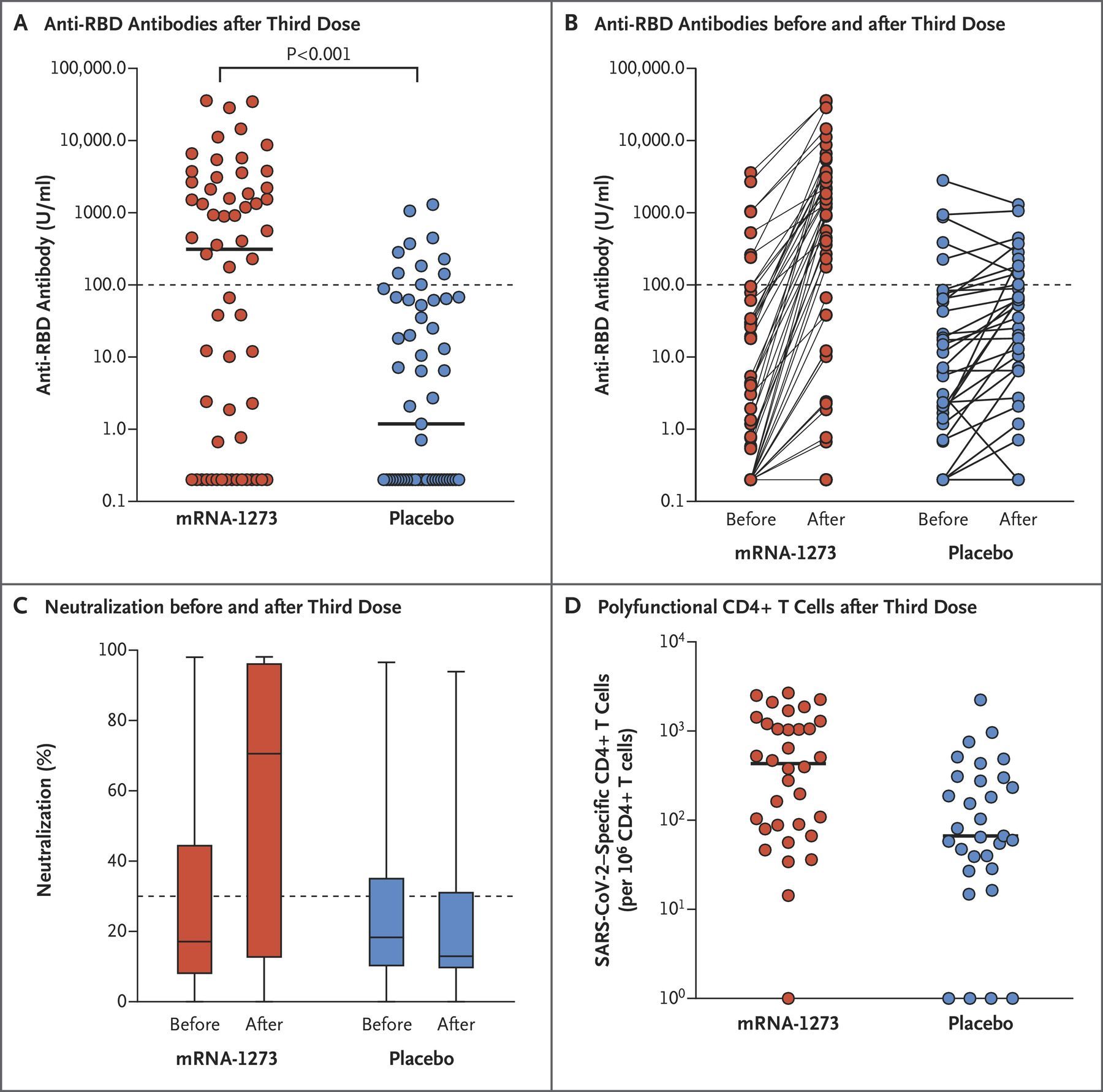
Source: NEJM
There is limited information about the risks of receiving an additional vaccine dose, including among the immunocompromised population. None of the COVID-19 vaccines authorized in the US or endorsed by WHO contain live virus. As noted above, there are theoretical concerns that vaccination may stimulate harmful immune responses but this has not been observed to date. In the aforementioned study on 30 solid organ transplant recipients who were given a third dose of Pfizer, Moderna or J&J vaccines, one participant experienced acute rejection of her transplanted heart seven days after her third vaccine dose, but it was not determined that the vaccine caused the rejection. Other studies in which immunocompromised persons have received third vaccine doses have not reported safety concerns. For example, in a study of the safety and impact on immune responses of a third Pfizer dose among people on hemodialysis, reactions reported after the third dose were similar to that of the two-dose series: fatigue and pain at injection site were the most commonly reported side effects, and most symptoms were mild to moderate.
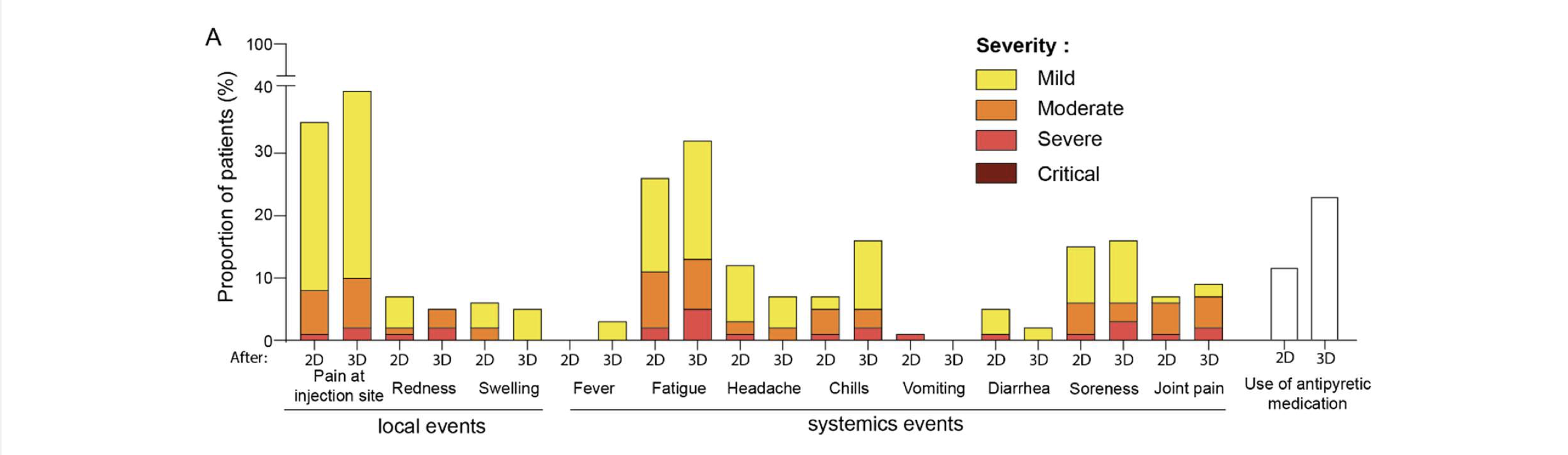
Source: medRxiv
There are currently no data on the effectiveness of third mRNA vaccine doses to prevent infection, severe disease or death due to COVID-19 among immunocompromised people. Available evidence has not revealed safety concerns associated with an additional dose, and data show that a third vaccine dose can boost the immune response, suggesting that there may be clinical benefit for immunocompromised persons who receive an additional dose.
Updated COVID-19 vaccination recommendations for immunocompromised persons
On August 12, 2021, the FDA amended emergency use authorizations (EUAs) for the Moderna and Pfizer vaccines to allow for the use of an additional (third) dose in some immunocompromised individuals. In both amendments, third doses were authorized in people with solid organ transplants or those “diagnosed with conditions that are considered to have an equivalent level of immunocompromise.”
On August 13, 2021, CDC made an interim recommendation for use of an additional dose of the Pfizer (for persons aged ≥12 years) or Moderna (for persons aged ≥18 years) vaccine after completion of an initial two-dose vaccine series for moderately to severely immunocompromised people. Some other countries also have recommended a third vaccine dose for immunocompromised persons, including France and Germany. The recommendation from CDC specifically includes people who:
- Have been receiving active cancer treatment;
- Received an organ transplant and are on immunosuppressants;
- Received a stem cell transplant within the past two years;
- Have a moderate or severe primary immunodeficiency;
- Have advanced HIV infection; or
- Are on active treatment with high-dose corticosteroids or other immunosuppressants.
CDC recommends that people talk to their health care provider about whether an additional dose is appropriate for them. Other recommendations include:
- The additional dose should be given at least 28 days after the second dose was administered, and
- The vaccine product given as a third dose should ideally match the product given for the first two doses; if the first vaccine product is unknown, either mRNA vaccine may be given as a third dose.
The optimal timing of a third dose is not clear. The current minimum 28-day recommendation was likely informed by laboratory and clinical data from vaccine clinical trials. Immunocompromised patients may receive vaccine doses on a wider range of schedules than immunocompetent people if vaccination is scheduled around periods of immunosuppression. The effects of mixing different vaccine products also are not clear. Data suggest that mixing and matching some vaccine products to complete a primary COVID-19 vaccine series yields immune responses at least as good as a homologous series without additional safety concerns. However, neither clinical effectiveness data on mixed-vaccine schedules nor data from immunocompromised populations are available.
CDC recommends against using results of immunologic (e.g., antibody) testing after completion of a two-dose series to guide decisions on third vaccine doses. There are no established immune correlates of protection against COVID-19, so the risk of infection cannot be quantified using lab data. In addition, there are no laboratory tests approved by FDA for the purpose of assessing post-COVID-19 vaccination immune response.
Another question that CDC’s interim recommendation did not address is whether immunocompromised persons who received the J&J vaccine should get an additional vaccine dose. CDC indicated that further data on antibody responses and protection among immunocompromised persons who received the J&J vaccine are needed, as well as data to inform which vaccine type might be recommended to follow the initial J&J shot.
Conclusion
Vaccination is the best available tool to prevent severe illness from COVID-19, including among immunocompromised people. CDC’s interim recommendation for a third mRNA dose for immunocompromised persons is supported by evidence of a potential to increase immune response and an acceptable safety profile, although the clinical benefit of an additional mRNA vaccine dose in this population is not yet known. Indeed, the clinical benefit of a third dose among immunocompromised persons must be established by data gathered after third doses are administered. Until we know more about the clinical benefit of a third dose, CDC’s guidance recommends that people who are immunocompromised continue to practice other infection prevention measures (masking, distancing, avoiding crowded indoor places), and that close contacts of immunocompromised persons be vaccinated.
Research Highlights
Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant — National Healthcare Safety Network, March 1–August 1, 2021
(MMWR, August 2021)
- This analysis used weekly data reported by 14,997 Centers for Medicaid & Medicare (CMS)-certified skilled nursing facilities or nursing homes to CDC’s National Healthcare Safety Network. The study was stratified into three time periods:
1) pre-Delta (March 1–May 9);
2) intermediate, the period when Delta circulation was documented but not predominant (May 10–June 20); and
3) Delta, when ≥50% of SARS-CoV-2 viruses sequenced were the Delta variant (June 21–August 1).
- The analysis included 10,428,783 weekly resident counts—including 1,531,446 (14.7%) unvaccinated residents, 5,174,098 (49.6%) fully vaccinated with Pfizer, and 2,633,700 (25.3%) fully vaccinated with Moderna (those who received a single dose of mRNA or the J&J vaccine or an unspecified vaccine were excluded from analysis).
- Overall, 6,879 COVID-19 cases were identified, including 2,113 (30.7%) in unvaccinated residents, 2,603 (37.8%) in residents fully vaccinated with Pfizer and 1,302 (18.9%) in residents fully vaccinated with Moderna.
- During the pre-Delta period, vaccine effectiveness against infection was 74.7% for any mRNA vaccine, 74.2% for Pfizer, and 74.7% for Moderna.
- During the Delta period, vaccine effectiveness against infection was 53.1% for any mRNA vaccine, 52.4% for Pfizer, and 50.6% for Moderna. Estimates from the Delta period were significantly lower than those from the pre-Delta period; estimates from the intermediate period were lower than those during the pre-Delta period, but this difference was not significant.
- The study did not assess vaccine effectiveness against severe disease or the effects of time since vaccination on effectiveness. Effectiveness of the J&J vaccine was not assessed.
Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults — United States, March–July 2021
(MMWR, August 2021)
- This study included adults aged ≥18 years admitted to 21 hospitals in 18 states from March 11–July 14, 2021. A case-control design was used to assess vaccine effectiveness against hospitalization among all patients and also among three high-risk sub-groups. Cases were hospitalized patients with COVID-like illness and a positive PCR or antigen test for SARS-CoV-2. Controls were hospitalized patients with at least one negative PCR test for SARS-CoV-2. Those who were incompletely vaccinated or had completed vaccination less than 14 days before illness onset were excluded.
- 3,089 patients were included in the final analysis (1,194 in the case group and 1,895 in the control group). Among cases, 11.8% were fully vaccinated, as were 52.1% of controls. Among 454 case-patient specimens with SARS-CoV-2 lineage determined, 242 (53.3%) were identified as Alpha and 74 (16.3%) as Delta.
- Overall vaccine effectiveness against hospitalization for COVID-19 was 86% over the full study period, including 90% among patients without immunocompromising conditions and 63% among patients with immunocompromising conditions. There was no statistically significant decrease in vaccine effectiveness during the study period in any high-risk subgroup.
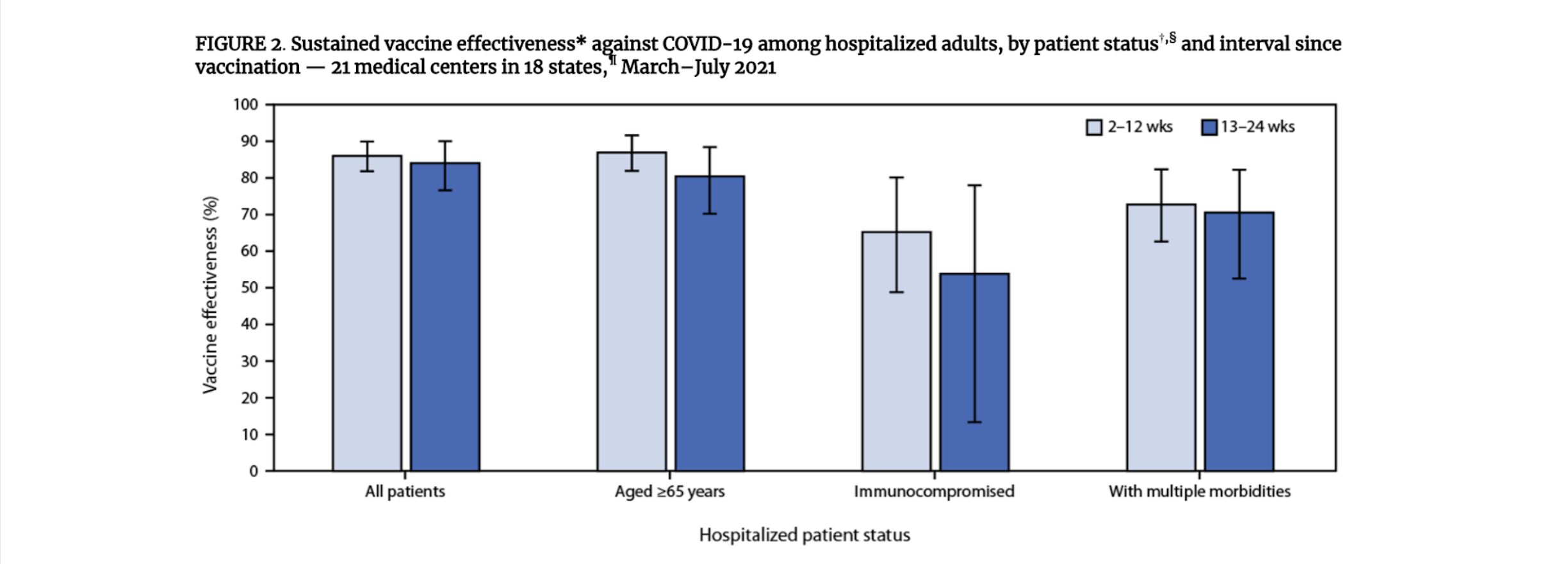
- Vaccine effectiveness among patients with illness onset during March–May was 87% (95% CI = 83%–90%), and among those with illness onset during June–July was 84% (95% CI = 79%–89%). Vaccine effectiveness was 86% (95% CI = 82%–90%) during the 2–12 weeks after the second vaccine dose and 84% (95% CI = 77%–90%) 13–24 weeks after the second dose.
- Limitations include: the follow-up period was limited to 24 weeks; effectiveness of the J&J vaccine was not assessed; and the effects of variants versus time on vaccine effectiveness could not be assessed.
Suggested citation: Cash-Goldwasser S, Jones SA, Bochner A, Cobb L and Frieden TR. In-Depth COVID-19 Science Review August 20, 2021. Resolve to Save Lives. 2021 August 20. Available from https://preventepidemics.org/covid19/science/review/





