Insight
Update on COVID-19 in Africa
Since the first COVID-19 case in Africa was recorded on Feb. 14, 2020 in Egypt, there have been more than 1.1 million reported confirmed cases and more than 26,000 deaths across the continent. This is a large total, but it is lower than the high figures many projected (1,2,3) at the outset of the pandemic. Despite having 17% of the global population, Africa has accounted for just 5% of global COVID-19 confirmed cases and 3% of global COVID-19 deaths.
Source: Our World in Data complete database
In this Insight, we examine epidemiological information to better understand what we know about COVID-19 in Africa and what critical information gaps remain.
Our analysis reveals that to address critical gaps in our knowledge of COVID-19 in Africa, we need: 1) Regularly updated testing data to understand limitations in case data; 2) Response data to understand if measures such as contact tracing are performing adequately; 3) Rigorously conducted serosurveys to estimate the true prevalence of disease and inform infection fatality rate (IFR) estimates 4) Weekly excess mortality data to understand the overall impact of the pandemic on total deaths; and 5) Ongoing scientific research to understand how different factors relevant to Africa interact with COVID-19 transmission and severity of illness.
In-depth
Herd immunity, reproduction numbers and human behavior during the COVID-19 pandemic
What is herd immunity and how does it develop?
In order for an infectious disease to spread in a community, susceptible people must come into contact with the causative pathogen and become infected. When a proportion of the community (the “herd”) becomes immune to a pathogen, the spread of disease becomes less likely because there is only a small proportion of susceptible people, and the whole community is protected, including those who are not immune themselves. That type of community protection may be referred to as “herd immunity.” Historically, this concept has been relevant to vaccination coverage targets. For example, despite the availability of an effective vaccine, maintaining sufficient herd immunity against measles to prevent outbreaks continues to be a global challenge.
Herd immunity can be the outcome of natural infections and/or vaccination of people within a population. At the individual level, immunity can result when the immune system, a highly complex array of organs, cells and proteins that fights infections, learns to recognize an infectious agent either through natural infection or vaccination. Although it is not yet clear what degree of individual protection is conferred by natural COVID-19 infection nor how long that immunity lasts, it is clear that natural infection can result in suffering and death. Thus, although there are similar questions about the degree and longevity of protection that could result from vaccination, vaccination is the preferred strategy to induce herd immunity. One goal of vaccination against COVID-19, when an effective vaccine becomes available, will be to attain enough herd immunity to limit or stop the spread of COVID-19. Infection-induced immunity may also play a role in that scenario, and infection-induced immunity may already be mitigating epidemic spread in some communities.
What amount of herd immunity is sufficient to control the spread of disease?
The proportion of the population that must be immune in order to stop an epidemic is different for each disease. For a very contagious disease, immunity among a higher proportion of the population is needed. Disease transmissibility, represented by the “basic reproduction number” (R0), is highly relevant when attempting to define the proportion of herd immunity that might control an epidemic. The R0 is equal to the number of secondary cases generated by a typical infectious individual when the entire population is susceptible to the disease, as is the case at the start of a new outbreak. Because R0 is a function of biological characteristics of the causative pathogen as well as human behavior, it can be influenced by many factors such as the duration of contagiousness after a person becomes infected, the rate of contact between people, and the likelihood of infection per contact between a susceptible person and an infectious person. For many infectious diseases, the R0 is cited as a range of numbers estimated using data from different populations in different settings. For example, estimates of the R0 for measles, one of the most highly contagious diseases, are highly variable but may be as high as 20. There also is a wide range of R0 estimates for COVID-19; because many estimates fall between 2 and 3, numbers in that range are frequently used in modeling studies, although some published estimates are much higher.
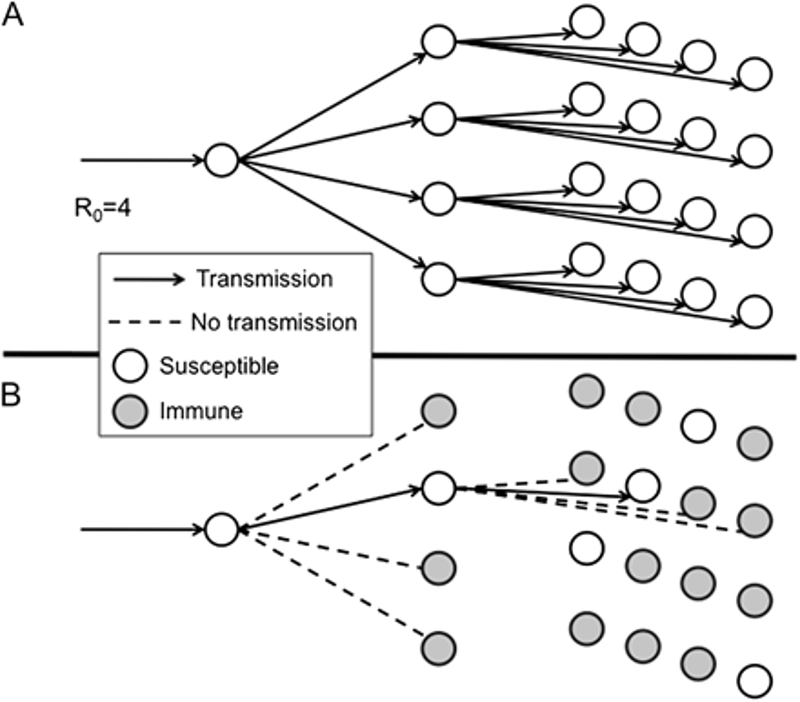
The “effective reproduction number” (Rt or Re), is defined as the number of secondary cases generated by a typical infectious individual during the progression of an epidemic, as people within the population change behavior and develop immunity. The following diagram illustrates two scenarios: a) transmission of a disease with an R0 of 4 in a fully susceptible population, and b) transmission of a disease with an R0 of 4 in a population in which three of every four people is immune; the Rt in this latter scenario is equal to 1.
Source: “Herd Immunity”: A Rough Guide
Both R0 and Rt are affected by the behavior of the population; public health and social measures designed to mitigate epidemic spread reduce the Rt. For COVID-19, the Rt has been a parameter of great global interest as governments have enforced, relaxed or reintroduced restrictions. For example, in April it was announced in Germany that the Rt had been successfully reduced to less than 1 in large part due to the lockdown. Estimates of the Rt in each U.S. state, available and updated in real time, show Rt fluctuations above and below 1. If the Rt falls to less than 1, an epidemic will theoretically stop, eventually, because each case generates less than one new case. The degree of herd immunity required to achieve an Rt below 1 may be referred to as the “herd immunity threshold.”
In the absence of a vaccine, can herd immunity control the spread of COVID-19?
Even if herd immunity thresholds have not been reached, some degree of herd immunity has potentially already played a role in curbing local COVID-19 epidemics by reducing the Rt. After initial reopenings in Florida, Arizona and New York City, there were spikes in cases corresponding to an increase of Rt in each location. But after those initial spikes waned, despite continued reopening, case counts decreased. Although public health and social measures were increasingly implemented and observed over time, the flattening of those epidemic curves despite reopening may have been due at least in part in some communities, to increases in population immunity. Even in places where some communities have high levels of infection, other communities have lower levels of infection; explosive spread in these latter groups remains a risk, even if the overall rate of infection in a community is relatively high. It has been suggested that discussing herd immunity thresholds outside of determining vaccination strategies is not useful and may be dangerous. If infection-induced immunity is incentivized, people may engage in risky behavior with the goal of becoming infected, or if there is a sense that a herd immunity threshold has been achieved, people may relax their adherence to public health and social measures, leading to more cases and deaths. In response, others have argued that some immunity will be a natural effect of the pandemic so it’s important to understand what effects immunity may have on the risk of further spread of COVID-19, and that perhaps lockdowns and other public health measures could be adjusted accordingly. For now, before an effective vaccine is available, the only road to herd immunity is through high numbers of cases, illnesses and deaths. As the effects of infection-induced herd immunity on the evolution of the pandemic are further studied, it is important that everyone—regardless of whether or not they have had COVID-19—observe the 3 W’s to reduce the risk of transmission: Wear a mask, Wash your hands and Watch your distance.
Traditional models for R0 assume that each contact between an infectious person and a susceptible person is equally likely to result in infection. Reality is more complicated. First, it is possible that different populations or subpopulations have different degrees of susceptibility to COVID-19 at baseline. Second, the degree of protection from COVID-19 after natural infection (or once a vaccine is publicly available, after vaccination) may differ among people. Third, public health and social measures (e.g., face masks, increased ventilation) designed to prevent transmission can significantly reduce the chance that an interaction between two people will lead to a new infection. Traditional models also assume that everyone in society mixes randomly with everyone else. This is not the case. Societal patterns and restrictions have influenced some subpopulations differently from others (e.g., people who can work from home and people who cannot). There are also striking behavioral differences between subpopulations. For example, older adults who are shielding from the virus mix differently with others than young adults who attend social events and mass gatherings. Seroprevalence studies, which measure the proportion of the population with antibodies to SARS-CoV-2, are illustrative of this. There are not only differences in seroprevalence between countries and U.S. states but also between zip codes, neighborhoods and communities.
For COVID-19, given an R0 of between 2 and 3, some public health experts have calculated that reaching a herd immunity threshold of 60% to 80% would bring the Rt down to less than 1. However, others have estimated that the herd immunity threshold may actually be much lower. In one study, which examined how the heterogeneity of the population in terms of social mixing rates affects infection-induced immunity, authors found that in an age-structured community with mixing rates fitted to social activity, a herd immunity threshold of around 43% would be sufficient to drive Rt from 2.5 down to 1. In other words, the levels of herd immunity necessary to control disease among the most socially active are not needed for the whole population. However, even if a lower herd immunity threshold may be sufficient to drive the Rt to less than 1, many seroprevalence surveys suggest that even modest thresholds have not been reached.
Guidance on how long people with COVID-19 should isolate
As COVID-19 spread rapidly around the world early this year, global and national health authorities quickly recommended that infected people be isolated to avoid the risk of transmitting the infection to others. The earliest recommendation from the World Health Organization (WHO) encouraged health officials to confirm the virus was gone—by documenting at least two negative test results—before releasing recovered patients from isolation. This conservative guidance was reasonable based on the high risk of severe and fatal illness associated with the novel coronavirus and how little was known about its transmission. But it was not perfect. To start, testing resources were in short supply and clinicians needed to conserve them to diagnose newly ill patients. In addition, maintaining isolation precautions either in hospital or in the community could adversely affect the health and well-being of recovering patients and families. Finally, some patients continued to test positive weeks after they had recovered and others reverted to positive even after having been cleared with two negative results. Such unexpected findings undermined trust in the testing technology and raised concern about the possibility that people could be repeatedly infected or reactivated after a short interval.
As the scientific understanding of COVID-19 improves, it has become clear that a positive test doesn’t necessarily mean that someone is able to infect others. Once infected with SARS-CoV-2, people begin to shed viral genetic material after a few days, usually starting before they develop symptoms. It is this component of the virus that is detected with the most reliable diagnostic test, called reverse transcriptase quantitative polymerase chain reaction (RT-qPCR). Patients can continue to shed viral genetic material, and produce positive results on RT-qPCR tests, even after they recover and no longer pose a risk to others. Researchers are piecing together a better picture of COVID-19 infection risk by tracking both viral genetic material and infectious virus in recovering patients. In a recent preprint release, investigators reported results from a systematic review of 14 studies that included viral cultures as a more direct measure of COVID-19 transmission potential than would be possible from RT-qPCR results alone. On the whole, these studies show that infectious virus concentrations in samples from COVID-19 patients start to decline shortly after the first symptoms appear and disappear entirely by day 10 in nearly all but some of the most severely ill patients.
Based on these findings and other evidence, the U.S. Centers for Disease Control and Prevention, WHO and other public health authorities have since May 2020 recommended a symptom-based approach for ending isolation and other precautions. For most people it is not necessary to test repeatedly until the results are negative. Instead, patients who develop COVID-19 should isolate until three conditions are met:
- 10 days have passed since the onset of their first symptoms;
- They have been without fever for at least 24 hours (without taking fever reducing medication); and
- Their other COVID-19 symptoms have improved (because the loss of taste and smell can take weeks to resolve, patients don’t need to wait for this symptom to improve or return to normal).
After that point, it is very unlikely that they could transmit the virus to others. People who have had very severe COVID-19 and those who are severely immunocompromised should consult their individual providers for specific advice about how long to maintain isolation and other precautions. Some of these patients may remain infectious for as long as 20 days from the onset of their symptoms and they may benefit from repeated testing. Finally, people who test positive for COVID-19 but never develop symptoms at all should isolate for a full 10 days from the time of their test. These recommendations are based on the best science available and will continue to be updated as more is learned. In addition, these recommendations are easier to follow than previous guidance and should reduce the amount of time that patients need to keep away from the people they want to protect.
Heating, ventilation and air conditioning (HVAC) systems and managing indoor air during the COVID-19 pandemic
COVID-19 has been shown to spread predominantly through close contact with someone who is actively infected with SARS-CoV-2, the virus that causes COVID-19. This spread is driven mainly by respiratory droplets: Particles of certain size that can carry virus from the lungs or airways. These particles are expelled from an infected person through breathing, coughing, sneezing or talking, and can infect a nearby person who breathes them in. Recently, smaller particles known as aerosols or droplet nuclei have been the subject of increased attention. This topic was covered in detail in an earlier Weekly Science Review.
There is evidence that live virus with the capacity to infect other cells, and in theory, other people, can be isolated from these smaller airborne particles. One reason this is particularly important is because the smaller aerosol particles can remain floating in the air longer than respiratory droplets, and could find their way into air ducts that spread them through indoor air in a large building. A recent preprint article documents the presence of viral genetic material from SARS-CoV-2 in a hospital heating, ventilation and air conditioning (HVAC) system, although researchers in this study did not test for live virus. What remains lacking is conclusive evidence that there would be enough virus present in these circumstances to put people at risk of infection if small aerosol virus particles are introduced into the air.
Despite the lack of extensive evidence that COVID-19 spreads through aerosol transmission, it is appropriate to take precautions. Several groups have produced guidelines on how to best manage indoor air to minimize risk of transmitting COVID-19 from any size particle. The U.S. Environmental Protection Agency has a site dedicated to indoor air and COVID-19. There, the agency outlines guidance for homes as well as commercial spaces, schools and offices on steps to take to improve indoor quality to minimize COVID-19 transmission.
In general, measures to improve indoor air quality focus on two processes: Air cleaning and ventilation. Air cleaning refers to the use of filters or air purifiers to capture indoor air contaminants, including virus-containing particles. Filters come with varying specifications that allow them to capture different particle sizes. Most homes and buildings have filters as part of their HVAC systems. High efficiency particulate air (HEPA) filters are one common type of regulated filter with an industry standard set at capturing at least 99.7% of airborne particles 0.3 micrometers in diameter. Their performance for capturing any sized contaminant particle approaches 99.97%. HEPA filters are not generally used in commercial HVAC systems because they significantly restrict air flow and burden system components. Most HVAC systems can be upgraded from standard filters to MERV-13, which trap 50% of airborne particles 0.3 micrometers in diameter. For reference, the U.S. CDC considers aerosols to be particles smaller than five micrometers and defines droplets to be larger than five micrometers. Some floor-standing or wall-mounted air purifiers use HEPA filtration as a way to remove contaminants from indoor environments. There are a variety of such purifiers on the market with varying degrees of evidence to support their effectiveness, and some have been the subject of lawsuits for making false health claims. Those that rely on HEPA filtration alone are safe to operate, and are effective in environments where windows are not opened. Those that rely on ionization, where positive or negative “charge” is added to air contaminants that are later trapped when they pass through metal plates should not be used as they create ozone, a potent respiratory irritant. Some devices pass air through UV light, where ionizing ultraviolet light radiation decontaminates air as it passes through a purifier, eliminating many contaminants including some microorganisms. UV disinfection works when there is sufficient energy transmitted for a sufficient time as air is exposed. UV has been demonstrated to be effective in reducing viral viability and transmission in health care settings. Despite widespread marketing of UV devices for HVAC systems, they have not been demonstrated to be effective for building-wide ventilation. Research around the efficacy and effectiveness of air purifiers other than those that use HEPA filters is lacking. Although portable air purifiers may remove contaminants from the air, thereby possibly reducing virus or other contaminants in the air, there is currently no conclusive evidence to suggest that they improve health or reduce the risk of COVID-19.
The EPA provides a guide to air cleaners including portable air purifiers here. It states clearly that these measures are not adequate at preventing poor health outcomes, and that they should be used in concert with evidence-based measures.
For both homes and commercial indoor spaces, the EPA says to consider using portable air purifiers or HVAC filters, especially if other options for improving ventilation are not possible. It emphasizes that alone, these measures are not adequate to protect people from getting COVID-19. In commercial spaces, the EPA recommends getting professional guidance to use the higher level of filter compatible with an existing HVAC system.
Ventilation refers to the introduction of fresh outdoor air into an indoor environment. Essentially through dilution, ventilation can lower the indoor concentration of contaminants, including virus-containing particles. In homes, ventilation can be improved by opening windows or doors so long as there is no safety risk to those inside the home, such as to small children. In some climates, this is not a feasible option due to excess heat and humidity, or cold and wet weather. Kitchen and bathroom exhaust fans can also aid in improving in-home ventilation by adding to air exchange and directly removing air and contaminants from a room. In larger commercial indoor spaces and homes, ventilation is typically managed by professionals who adjust the HVAC system. In general, ventilation can be improved by increasing the proportion of replacement air that is drawn from fresh air sources. Although federal entities recommend minimizing air recirculation and maximizing the proportion of outside air use in these types of spaces, systems generally have upper limits to the proportion of fresh air they can draw. Additional guidance on ventilation and COVID-19 from the EPA can be found here.
Some concern has risen about the role of air conditioning units and HVACs with respect to their potential to facilitate the spread COVID-19. In a room with recirculated air where someone with COVID-19 is expelling virus by talking, sneezing or coughing, it has been shown that virus particles riding on droplets can be propelled across a room by way of a draft or air current. Whether this can happen for aerosol-sized particles is not known. In homes, transmission related to the HVAC system should not be a concern. If you are living with a person infected with COVID-19, close contact with that person is the risk factor, not the HVAC system. In commercial spaces, it may be a concern, but there is a missing link: Definitive evidence that this type of transmission, though plausible, is a major source of disease transmission. Nevertheless, some of the precautions to minimize risk from this type of transmission have been discussed here.
Air cleaning and ventilation, both in homes and large indoor spaces, come with costs. There are material costs for supplies such as filters and portable units, as well as energy costs and environmental harms for running these systems in a less energy-efficient manner needed to adjust the temperature and humidity of outside air for added ventilation. With increasing concern about indoor air and transmission of COVID-19, individuals and facility managers may consider consulting HVAC professionals about appropriate changes they can make to manage air quality in indoor spaces as a precautionary measure. Wearing masks substantially reduces the generation and introduction of respiratory droplets and aerosols, and reduces the velocity with which they are exhaled, limiting the distance they travel before being subject to the effects of gravity. Mask use, washing hands, and watching distance remain the mainstays of reducing the risk of COVID-19 transmission both indoors and outdoors.
FAQs
What is saliva-based testing for COVID-19?
On Aug. 15 the U.S. Food and Drug Administration (FDA) issued an emergency use authorization for a saliva-based COVID-19 test developed by the Yale School of Public Health. The test, called SalivaDirect, is a lab-based diagnostic test that uses polymerase chain reaction (PCR) technology to detect genetic material from SARS-CoV-2, the virus that causes COVID-19. It differs from more widely available PCR tests in several ways. It does not require a special container with preservatives to protect the sample while it is in transport to a lab. It does not require a special swab to obtain the specimen from deep in the back of the nose, alleviating supply chain issues and decreasing patient discomfort. And, the process to prepare the sample for testing has fewer steps, takes less time, and does not require special chemicals or dedicated new machines. For all of these reasons, it will be a cheaper testing option, and the creators have made their innovative protocol “open-source,” or publicly available to interested laboratories. In preliminary testing published in a preprint article, the SalivaDirect test has a high agreement (>94%) with swab PCR tests taken from the same patients.
As of Aug. 21, SalivaDirect is the fifth saliva test to receive an emergency use authorization from the FDA for COVID-19 testing, but it is unique in that it does not require special collection tubes, thereby creating an opportunity to further decrease cost and logistic or supply chain issues. None of these saliva tests are “rapid” tests that can be completed in a provider’s office, and they all still need a sample to be collected and sent to a specialized laboratory for processing, however there is no proprietary equipment needed to run the test. Making the protocol open source will allow laboratories to adapt it to the PCR kits and machines they already have. Authorities hope that saliva-based testing will add to the country’s overall testing capacity, reduce test turnaround times, and decrease stress on supply chains for testing materials that are in short supply. However, it is likely less sensitive (able to generate a positive result when someone is infected) than PCR testing, and actual experience under program conditions is required to determine how much less sensitive it will be.
Weekly Research Highlights
Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19)
(JAMA Cardiology, July 27)
- Cardiac damage was measured based on 1) blood tests for cardiac markers such as high sensitivity troponin and 2) cardiac magnetic resonance imaging findings. The most common abnormality was myocardial inflammation (60/100 patients).
- Cardiac damage was significantly greater in the 100 patients recovered from COVID-19 compared to both healthy and risk-factor-matched controls.
- While suggestive, the findings of this study should be interpreted with caution. The sample size was small and patients in the study all had relatively recent COVID-19 infection (time since diagnosis ranged from <one month to ~115 days) so it is possible that the cardiac damage will resolve over time. Finally, while the article compared the study population to healthy controls and people with similar cardiac risk factors who did not have COVID-19, it didn’t compare to people recovering from other viral infections.
Disparities in Incidence of COVID-19 Among Underrepresented Racial/Ethnic Groups in Counties Identified as Hotspots During June 5–18, 2020 — 22 States, February–June 2020
(MMWR, Aug. 21)
- Researchers used data from 79 U.S. counties in 22 states where case and race/ethnicity data were readily available to identify disparities in COVID-19 incidence, defined as a “difference of ≥5% between the proportion of cases and the proportion of the population or a ratio ≥1.5 for the proportion of cases to the proportion of the population for underrepresented racial/ethnic groups in each county.
- Disparities were apparent in 96% of the counties examined. Disparities were most common among people identified as Latinx, with nearly 75% of counties with disparities showing higher incidence in this community. Blacks had a disproportionately higher incidence in 28% of the counties with disparity.
- This study did not examine disparities in COVID-19 mortality by race due to insufficient data. Some hot spot counties were not examined because they had too much missing data for race/ethnicity. Although hot spots were identified during a two-week period in June, cumulative COVID-19 incidence was analyzed to identify disparities.
Limited Secondary Transmission of of SARS-CoV-2 in Child Care Programs — Rhode Island, June 1–July 31, 2020
(MMWR, early release Aug. 21)
- By July 31, 666 of Rhode Island’s 891 child care programs had qualified to reopen, taking additional steps to minimize risk of COVID-19 for children and staff alike. Attendance was first limited to groups of 12, and, subsequently, 20 people.
- Following reopening on June 1, the state’s health department investigated 101 possible cases of COVID-19 thought to be associated with child care programs. Among these, 49 were ruled out after a negative test for SARS-CoV-2, the virus that causes COVID-19, while 33 were ruled in based on a positive test. The remaining 19 were classified as probable cases. Of the 52 confirmed and probable cases, 30 were among children and 20 were among adults, including 18 teachers and two parents. Isolation of cases and quarantining of contacts was performed. Secondary transmission was identified in four child care centers with 10 cases occurring among contacts.
- Adherence to mitigation measures, both in child care programs and the surrounding community, as well as timely public health action to interrupt disease transmission, are necessary to safely reopen child care programs. Reduced-size and physically separated groupings, mask use by adults, and enhanced cleaning procedures allowed child care programs to safely reopen, and timely response from the health department was effective in containing transmission when cases were identified.
Suggested citation: Cash-Goldwasser S, Kardooni S, Cobb L, Bochner A, Bradford E and Shahpar C. In-Depth COVID-19 Science Review August 15 – 21, 2020. Resolve to Save Lives. 2020 August 26. Available from https://preventepidemics.org/covid19/science/review/